Functionalized Silica Materials: Preparation and Applications
A special issue of Materials (ISSN 1996-1944). This special issue belongs to the section "Materials Chemistry".
Deadline for manuscript submissions: closed (20 March 2023) | Viewed by 11342
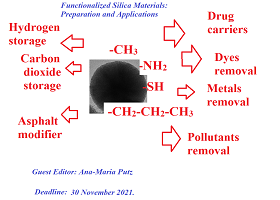
Image courtesy of Dr. Ana-Maria Putz
Special Issue Editor
Interests: functionalized silica materials; iron oxide silica nanocomposites; adsorption; drug loading; ionic liquids
Special Issues, Collections and Topics in MDPI journals
Special Issue Information
Dear Colleagues,
This Special Issue is intended to present studies on the preparation and characterization of controlled functionalized porous materials with tailored properties that can be used in different applications. The functionalization of the porous silica materials has been used as a method in view of tuning the physical and chemical properties to be applied such as drug carriers, gas storage materials, water pollutants adsorbents, or as asphalt modifier. The present Special Issue aims to gather some of the current challenging research explorations in the use of different functional groups, the presence of organic groups within the pores or of the amount of a certain functional group which will be attached at the material surface by one or another functionalization procedure, and in how all these separate or combined effects will merge together to promote the resulting material for a certain application. The topics of interest include but are not limited to: preparation of functionalized porous materials via co-condensation or post grafting methods; structural and morphological characterization of these materials with tailored properties; functionalized silica materials for hydrogen, methane, or carbon dioxide storage; functionalized porous silica materials as drug carriers; functionalized mesoporous silica materials used for removal of dyes, heavy metals, or other pollutants from waste water; functionalized silica materials used as an asphalt modifier.
Dr. Ana-Maria Putz
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Materials is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- functionalized silica materials
- co-condensation
- post-grafting
- drug loading
- gas adsorption
- pollutant removal






