Electroreduction of CO2: Novel Device and Engineering Innovation
A special issue of Processes (ISSN 2227-9717). This special issue belongs to the section "Chemical Processes and Systems".
Deadline for manuscript submissions: closed (20 July 2023) | Viewed by 8657
Special Issue Editors
Interests: CO2 utilization; multiphase reactor design; CFD simulation; interfacial engineering; process intensification
Interests: CO2 electroreduction; water splitting; electrocatalysis; material science; interface science
Special Issue Information
Dear Colleagues,
CO2 reduction through the electrochemical pathway has attracted considerable attention as a result of its unique advantages: (1) CO2 can be reduced by electrons instead of hydrogen; (2) the electrocatalytic process can take place under mild conditions; (3) the reduction products can be adjusted by the redox potential, reaction temperature, electrolytes, etc. Electrochemical CO2 reduction could be achieved through multiple electron transfer in an aqueous solution with appropriate electrocatalysts to produce different products, such as carbon monoxide, methane, ethylene, and even liquid products (formic acid, methanol, and ethanol). The success of the electrochemical approach depends upon researchers’ efforts to increase the Faraday efficiency and current density to optimize the reaction rate and product selectivity. In these reactions, water-based electrolytes act both as the proton source and the ion conductive medium. However, the solubility of CO2 in water is limited, leading to a constrained mass transfer rate and limited current density. Therefore, it is critical to design efficient devices to facilitate the controlling steps in line with new engineering principles.
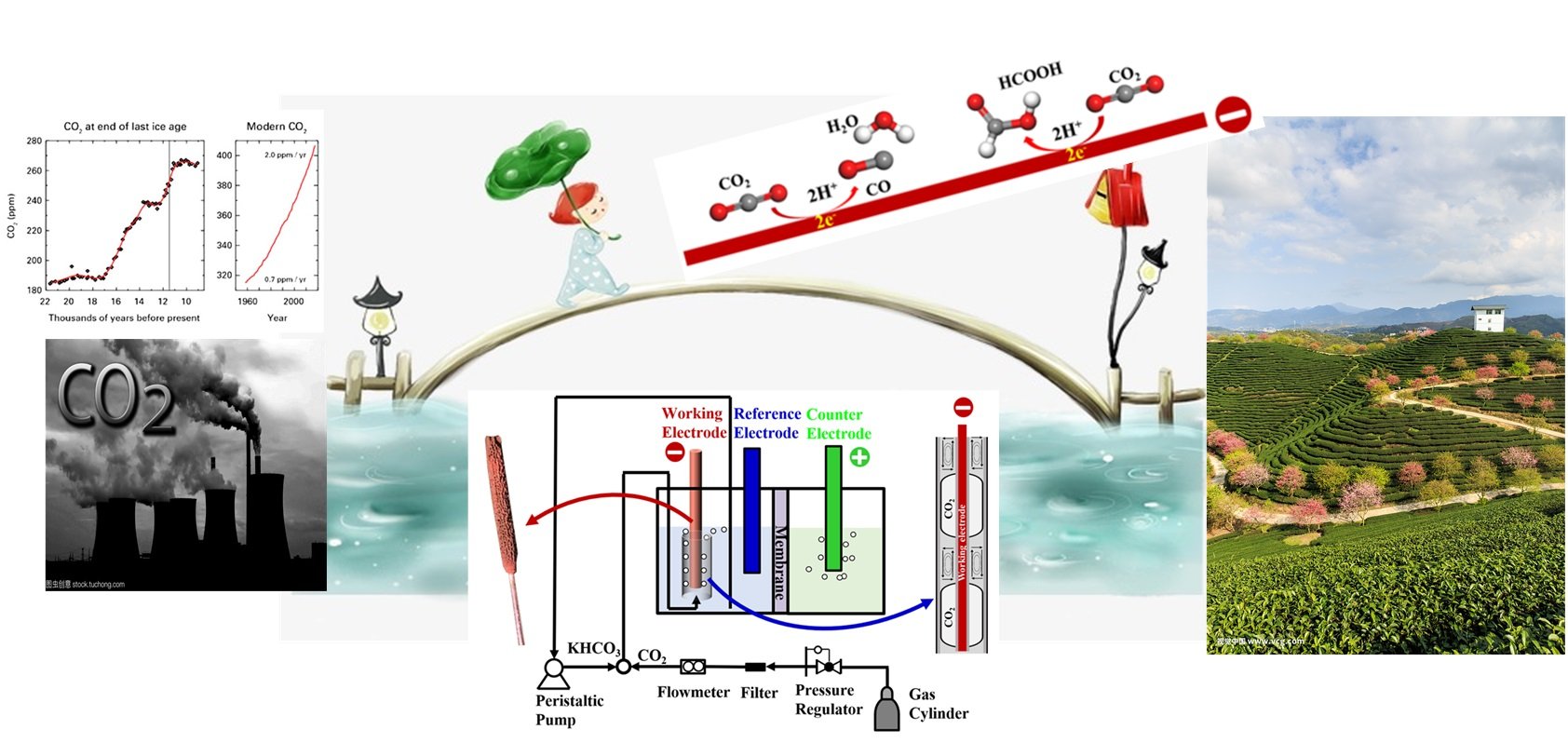
In recent years, different devices have been developed with respect to different engineering problems, such as the gas diffusion electrode to resolve the issue of low gas solubility and Taylor flow to ensure gas/liquid mass transfer without agitation. Such progress are very promising in promoting CO2 electroreduction from the catalyst development stage to commercial application.
This Special Issue, entitled “CO2 Reduction: Novel Device and Engineering Innovation”, is dedicated to providing a platform to highlight the recent advances in this field. Potential topics include, but are not limited to, the following:
- Engineering innovations in solving the problems in the CO2 electroreduction process;
- Design of novel devices in CO2 electroreduction;
- Investigations into the operation of different kinds of CO2 electroreduction devices;
- Gas–liquid–solid interface engineering: simulation and experiments.
Prof. Dr. Zhen-Min Cheng
Prof. Dr. Bo Zhang
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Processes is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2400 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- CO2 conversion
- electroreduction
- device design
- gas/liquid flow
- CFD simulation
- interfacial engineering
- process intensification






