Design, Synthesis, and Biological Evaluation of Novel Benzofuran Derivatives Bearing N-Aryl Piperazine Moiety
Abstract
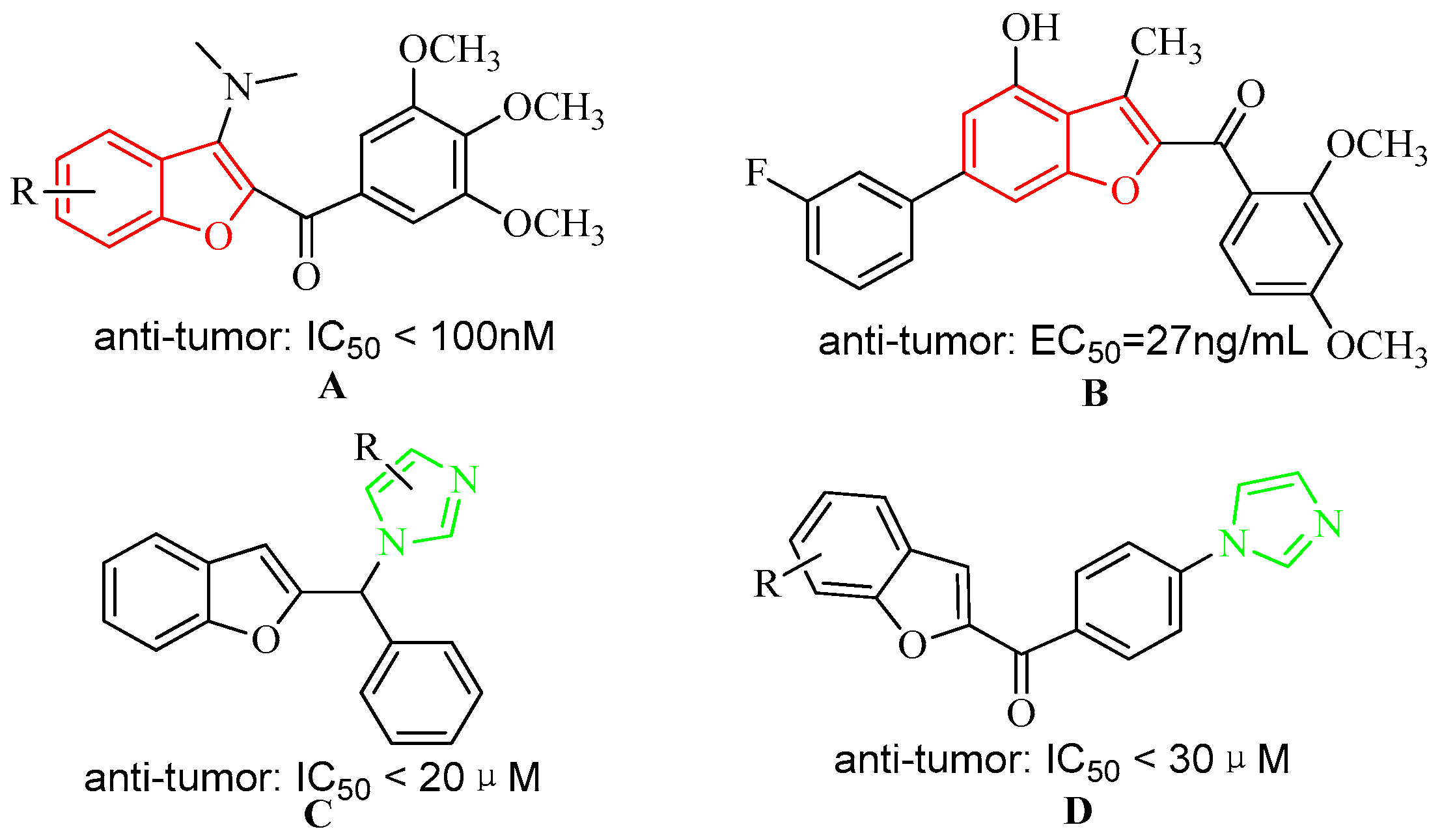
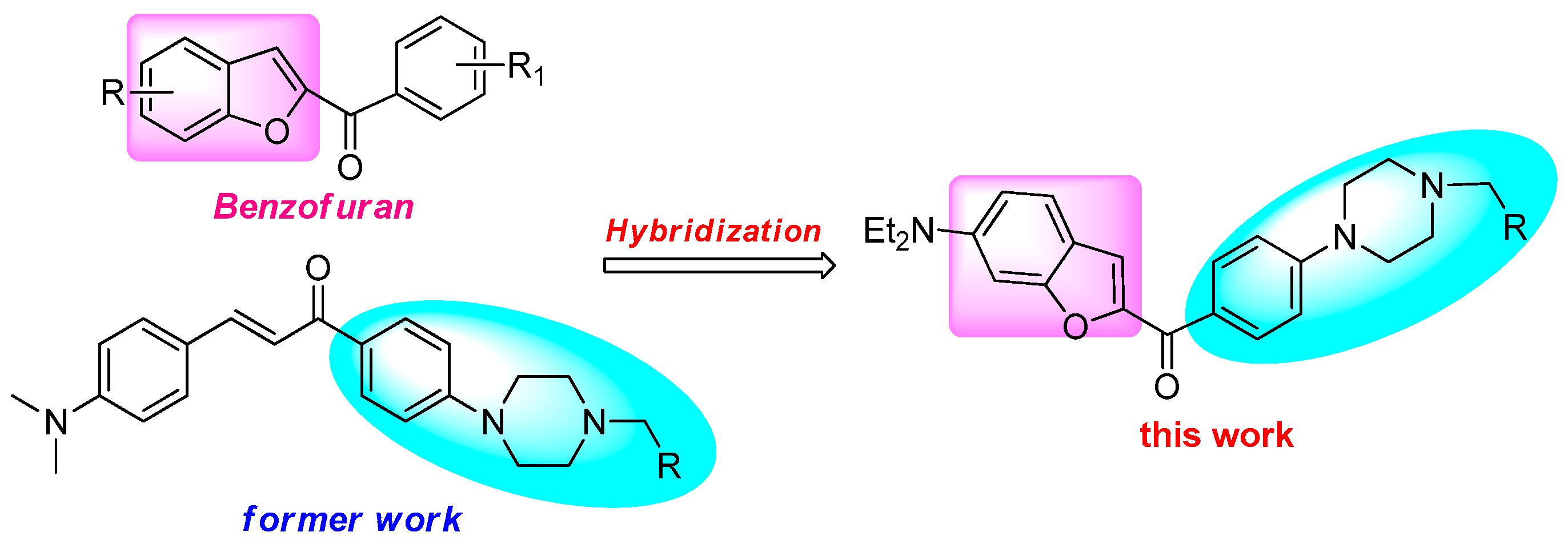
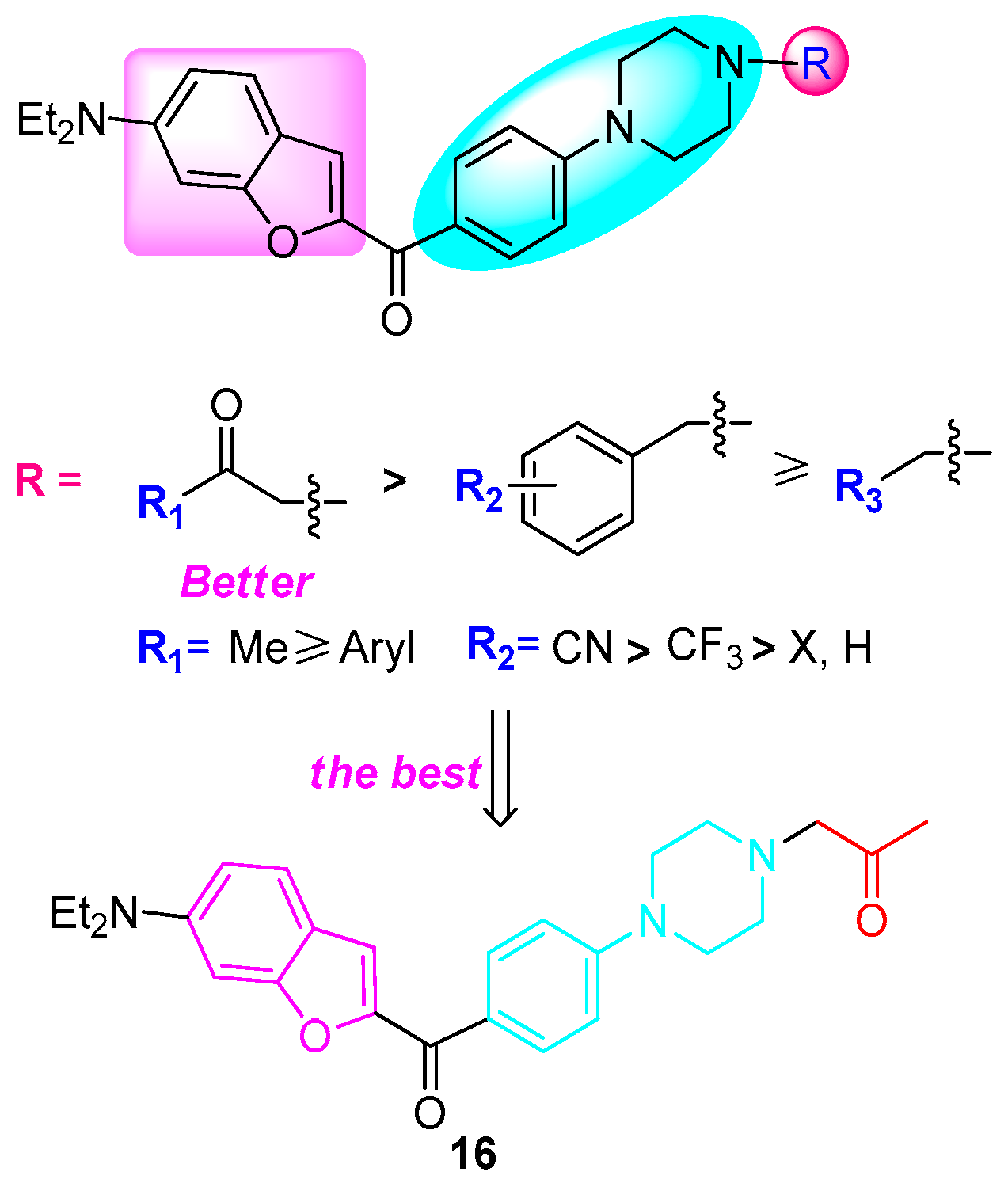
:1. Introduction
2. Results
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Anti-Inflammatory Activity
2.2.2. Anticancer Activity
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.2.1. General Procedure
3.2.2. The Character of All Compounds
3.3. Biological Activity Experiments
3.3.1. Anti-Inflammatory Activity
3.3.2. Antitumor Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xue, S.T.; Guo, H.F.; Liu, M.J.; Jin, J.; Ju, D.H.; Liu, Z.Y.; Li, Z.R. Synthesis of a novel class of substituted benzothiophene or benzofuran derivatives as BMP-2 up-regulators and evaluation of the BMP-2-up-regulating effects in vitro and the effects on glucocorticoid-induced osteoporosis in rats. Eur. J. Med. Chem. 2015, 96, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.S.; Abou-Seri, S.M.; Kamel, G.; Ali, M.M. Celecoxib analogs bearing benzofuran moiety as cyclooxygenase-2 inhibitors: Design, synthesis and evaluation as potential anti-inflammatory agents. Eur. J. Med. Chem. 2014, 76, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Carrër, A.; Brinet, D.; Florent, J.C.; Rousselle, P.; Bertounesque, E. Palladium-Catalyzed Direct Arylation of Polysubstituted Benzofurans. J. Org. Chem. 2012, 77, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Meshram, H.M.; Reddy, B.C.; Prasad, B.R.V.; Goud, P.R.; Kumar, G.S.; Kumar, R.N. DABCO-Promoted Efficient and Convenient Synthesis of Benzofurans. Synth. Commun. 2012, 42, 1669–1676. [Google Scholar] [CrossRef]
- Kumaraswamy, G.; Ramakrishna, G.; Raju, R.; Padmaja, M. An expedient synthesis of enantioenriched substituted (2-benzofuryl)arylcarbinols via tandem Rap–Stoermer and asymmetric transfer hydrogenation reactions. Tetrahedron 2010, 66, 9814–9818. [Google Scholar] [CrossRef]
- Watanabe, H.; Kawasaki, A.; Sano, K.; Ono, M.; Saji, H. Synthesis and evaluation of copper-64 labeled benzofuran derivatives targeting β-amyloid aggregates. Bioorg. Med. Chem. 2016, 24, 3618–3623. [Google Scholar] [CrossRef] [PubMed]
- Na, M.; Hoang, D.M.; Njamen, D.; Mbafor, J.T.; Fomum, Z.T.; Thuong, P.T.; Ahn, J.S.; Oh, W.K. Inhibitory effect of 2-arylbenzofurans from Erythrina addisoniae on protein tyrosine phosphatase-1B. Bioorg. Med. Chem. Lett. 2007, 17, 3868–3871. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liang, Z.; Xu, H.; Mou, Y.; Guo, C. Design, Synthesis and Cytotoxicity of Novel Dihydroartemisinin-Coumarin Hybrids via Click Chemistry. Molecules 2016, 21, 758–772. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Xu, H.; Tian, Y.; Guo, M.; Su, X.; Guo, C. Design, Synthesis and Antifungal Activity of Novel Benzofuran-Triazole Hybrids. Molecules 2016, 21, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Liu, L.X.; Li, Y.; Sun, C.J.; Chen, W.; Li, L.; Zhang, H.B.; Yang, X.D. Design, synthesis and biological evaluation of novel hybrid compounds of imidazole scaffold-based 2-benzylbenzofuran as potent anticancer agents. Eur. J. Med. Chem. 2013, 62, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Vinh, T.K.; Yee, S.W.; Kirby1, A.J.; Nicholls1, P.J.; Simons, C. 1-[(Benzofuran-2-yl) phenylmethyl] triazoles as steroidogenic inhibitors: Synthesis and in vitro inhibition of human placental CYP19 aromatase. Anti-Cancer Drug Des. 2001, 16, 217–225. [Google Scholar]
- Romagnoli, R.; Baraldi, P.G.; Sarkar, T.; Carrion, M.D.; Cruz-Lopez, O.; Cara, C.L.; Tolomeo, M.; Grimaudo, S.; Cristina, A.D.; Pipitone, M.R.; et al. Synthesis and biological evaluation of 2-(3′,4′,5-trimethoxy benzoyl)-3-N,N-dimethylamino benzo[b]furan derivatives as inhibitors of tubulin polymerization. Bioorg. Med. Chem. 2008, 16, 8419–8426. [Google Scholar] [CrossRef] [PubMed]
- Pevet, I.; Brulé, C.; Tizot, A.; Gohier, A.; Cruzalegui, F.; Boutin, J.A.; Goldstein, S. Synthesis and pharmacological evaluation of thieno[2,3-b]pyridine derivatives as novel c-Src inhibitors. Bioorg. Med. Chem. 2011, 19, 2517–2528. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Wan, W.C.; Deng, X.Y.; Li, Y.; Yang, L.J.; Li, L.; Zhang, H.B. Design, synthesis and cytotoxic activities of novel hybrid compounds between 2-phenylbenzofuran and imidazole. Bioorg. Med. Chem. Lett. 2012, 22, 2726–2729. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.W.; Wan, C.P.; Jiang, Y.; Guo, W.L.; Rao, G.X. Synthesis and Anti-tumor activity in vitro of N-hetercycle substitued benzofuran derivatives. J. China Pharm. Univ. 2015, 46, 58–61. [Google Scholar]
- Vergelli, C.; Ciciani, G.; Cilibrizzi, A.; Crocetti, L.; Mannelli, L.D.C.; Ghelardini, C.; Guerrini, G.; Iacovone, A.; Giovannoni, M.P. Synthesis of five and six-membered heterocycles bearing an arylpiperazinylalkyl side chain as orally active antinociceptive agents. Bioorg. Med. Chem. 2015, 23, 6237–6245. [Google Scholar] [CrossRef] [PubMed]
- Biswas, N.N.; Kutty, S.K.; Iskander, G.M.; Mielczarek, M.; Bhadbhade, M.M.; Gardner, C.R.; Black, D.S.; Kumar, N. Synthesis of brominated novel N-heterocycles: New scaffolds for antimicrobial discovery. Tetrahedron 2016, 72, 539–546. [Google Scholar] [CrossRef]
- Chaudhary, P.; Kumar, R.; Verma, A.K.; Singh, D.; Yadav, V.; Chhillar, A.K.; Sharma, G.L.; Chandra, R. Synthesis and antimicrobial activity of N-alkyl and N-aryl piperazine derivatives. Bioorg. Med. Chem. 2006, 14, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Prosser, A.R.; Liottaa, D.C.; Wilson, L.J. Discovery of novel N-aryl piperazine CXCR4 antagonists. Bioorg. Med. Chem. Lett. 2015, 25, 4950–4955. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.W.; Zheng, X.; Lin, Y.P.; Hu, C.Y.; Wang, X.L.; Wan, C.P.; Rao, G.X. Design, synthesis and anticancer activity of novel hybrid compounds between benzofuran and N-aryl piperazine. Bioorg. Med. Chem. Lett. 2016, 26, 3421–3424. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.W.; Zheng, X.; Qi, Y.; Zhang, M.D.; Huang, Y.; Wan, C.P.; Rao, G.X. Synthesis and biological evaluation of novel hybrid compounds between chalcone and piperazine as potential antitumor agents. RSC Adv. 2016, 6, 7723–7727. [Google Scholar] [CrossRef]
- Mao, Z.W.; Zheng, X.; Lin, Y.P.; Qi, Y.; Hu, C.Y.; Wan, C.P.; Rao, G.X. Concise synthesis and biological evaluation of chalcone derivatives bearing N-heterocyclic moieties. Heterocycles 2016, 92, 1102–1110. [Google Scholar]
- Sample Availability: Samples of the compounds 3–25 are available from the authors.

| Compound | R | m.p. (°C) | Yields (%) a |
|---|---|---|---|
| 5 |  | 171–173 | 51 |
| 6 |  | 171–173 | 57 |
| 7 |  | 185–186 | 68 |
| 8 |  | 174–176 | 83 |
| 9 |  | 176–178 | 81 |
| 10 |  | 179–181 | 83 |
| 11 |  | 186–188 | 79 |
| 12 |  | 180–182 | 76 |
| 13 |  | 178–180 | 82 |
| 14 |  | 187–189 | 81 |
| 15 |  | 156–158 | 91 |
| 16 |  | 161–163 | 86 |
| 17 |  | 188–190 | 82 |
| 18 |  | 202–204 | 70 |
| 19 |  | 192–193 | 84 |
| 20 |  | 194–196 | 75 |
| 21 |  | 198–200 | 83 |
| 22 |  | 181–183 | 85 |
| 23 |  | 204–206 | 77 |
| 24 |  | 184–186 | 82 |
| 25 |  | 135–137 | 86 |
| Compound | NO Generation (IC50, μM) a | Compound | NO Generation (IC50, μM) a |
|---|---|---|---|
| 5 | 14.12 | 16 | 5.28 |
| 6 | 34.24 | 17 | 25.40 |
| 7 | >40 | 18 | 6.53 |
| 8 | >40 | 19 | >40 |
| 9 | 18.52 | 20 | >40 |
| 10 | >40 | 21 | >40 |
| 11 | >40 | 22 | 9.13 |
| 12 | >40 | 23 | 23.56 |
| 13 | 23.06 | 24 | 18.37 |
| 14 | 20.27 | 25 | >40 |
| 15 | 31.68 |
| Compound | Cell Lines (IC50, μM) a | ||
|---|---|---|---|
| A549 | Hela | SGC7901 | |
| 5 | >40 | >40 | 27.24 |
| 6 | >40 | 32.53 | >40 |
| 7 | >40 | >40 | >40 |
| 8 | >40 | >40 | >40 |
| 9 | 19.27 | >40 | >40 |
| 10 | >40 | >40 | >40 |
| 11 | 16.14 | 8.57 | >40 |
| 12 | >40 | 25.14 | 16.27 |
| 13 | >40 | >40 | 25.04 |
| 14 | >40 | 33.24 | >40 |
| 15 | >40 | 22.36 | 30.43 |
| 16 | 0.12 | 26.32 | 2.75 |
| 17 | 27.82 | >40 | 15.41 |
| 18 | 19.34 | >40 | 23.92 |
| 19 | 6.25 | 18.71 | 36.23 |
| 20 | 8.11 | 28.74 | >40 |
| 21 | 23.22 | 15.35 | >40 |
| 22 | 26.07 | >40 | >40 |
| 23 | 34.13 | 12.68 | 7.45 |
| 24 | >40 | 27.58 | >40 |
| 25 | >40 | 26.22 | >40 |
| DDP | 11.54 | 20.52 | 12.44 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zheng, X.; Gao, H.; Wan, C.; Rao, G.; Mao, Z. Design, Synthesis, and Biological Evaluation of Novel Benzofuran Derivatives Bearing N-Aryl Piperazine Moiety. Molecules 2016, 21, 1684. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21121684
Ma Y, Zheng X, Gao H, Wan C, Rao G, Mao Z. Design, Synthesis, and Biological Evaluation of Novel Benzofuran Derivatives Bearing N-Aryl Piperazine Moiety. Molecules. 2016; 21(12):1684. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21121684
Chicago/Turabian StyleMa, Yulu, Xi Zheng, Hui Gao, Chunping Wan, Gaoxiong Rao, and Zewei Mao. 2016. "Design, Synthesis, and Biological Evaluation of Novel Benzofuran Derivatives Bearing N-Aryl Piperazine Moiety" Molecules 21, no. 12: 1684. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21121684






