Synthesis and Antitumor Evaluation of Novel 5-Hydrosulfonyl-1H-benzo[d]imidazol-2(3H)-one Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Anti-Cancer Activity
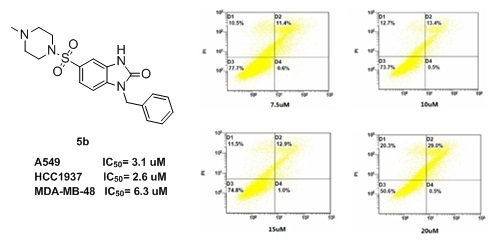
2.3. Flow Cytometry
2.4. Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.2.1. N-Benzyl-2-Nitroaniline (2a)
3.2.2. N-Benzyl-4-(morpholinosulfonyl)-2-nitroaniline (3a)
3.2.3. N′-Benzyl-4-(morpholinosulfonyl)benzene-1,2-diamine (4a)
3.2.4. Benzyl-5-(morpholinosulfonyl)-1H-benzo[d]imidazol-2(3H)-one (5a)
3.3. Cell Proliferation Assay
3.4. Flow Cytometry
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goss, P.E.; Strasser-Weipp, K.; Lee-Bychkovskyet, B.L. Challenges to effective cancer control in China, India, and Russia. Lancet. Oncol. 2014, 15, 489–538. [Google Scholar] [CrossRef]
- Bray, F.; Jemal, A.; Greyet, N. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet. Oncol. 2012, 13, 790–801. [Google Scholar] [CrossRef]
- Pettit, G.R.; McNulty, J.; Herald, D.L.; Doubek, D.L.; Chapuis, J.C.; Schmidt, J.M.; Tackett, L.P.; Boyd, M.R. Antineoplastic Agents. 362. Isolation and X-ray crystal structure of dibromophakellstatin from the Indian Ocean Sponge Phakellia Mauritiana. J. Nat. Prod. 1997, 60, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Cafieri, F.; Fattorusso, E.; Mangoni, A. Longamide and 3,7-dimethylisoguanine, two novel alkaloids from the marine sponge Agel aslongissima. Tetrahedron Lett. 1995, 36, 7893–7896. [Google Scholar] [CrossRef]
- Liu, W.; Lau, F.; Liu, K. Benzimidazolones: A New class of selective peroxisome proliferator-activated receptor γ (PPARγ) modulators. J. Med. Chem. 2011, 54, 8541–8554. [Google Scholar] [CrossRef] [PubMed]
- Budzik, B.; Garzya, V.; Shi, D. Selective, novel N-substituted benzimidazolones as potent, selective, CNS-penetrant, and orally active M1 mAChR agonists. ACS Med. Chem. Lett. 2010, 1, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Li, S.K.; Ji, Z.Q.; Zhang, J.W. Synthesis of 1-Acyl-3-isopropenylbenzimidazolone derivatives and their activity against botrytis cinerea. J. Agric. Food. Chem. 2010, 58, 2668–2672. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, N.C.; Stefan, Z.; Francoise, D. Synthetic organic pigments of the 20th and 21st century relevant to artist’s paints: Raman spectra reference collection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 505–524. [Google Scholar] [CrossRef] [PubMed]
- Bruncko, M.; Tahir, S.K.; Song, X. N-Aryl-benzimidazolones as novel small molecule HSP90 inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 7503–7506. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Robinson, R.G.; Fu, S. Rapid assembly of diverse and potent allosteric Akt inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 2211–2214. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, C.W.; Zhao, Z.; Leister, W.H. Allosteric Akt (PKB) inhibitors: Discovery and SAR of isozyme selective inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmi, G.A.; Chen, C.S.; Hakimelahi, G.H. Design, synthesis, and cytotoxicity of 4-sulfonamide substituted benzamidobenzimidazolones and an acyl benzimidazolone. J. Iran. Chem. Soc. 2005, 2, 124–134. [Google Scholar] [CrossRef]
- Ballante, F.; Caroli, A.; Wickersham, R.B. Hsp90 inhibitors, part 1: Definition of 3-D QSAutogrid/R models as a tool for virtual screening. J. Chem. Inf. Model. 2014, 54, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Rzasa, R.M.; Kaller, M.R.; Liu, G. Structure-activity relationships of 3,4-dihydro-1H-quinazolin-2-one derivatives as potential CDK5 inhibitors. Bioorg. Med. Chem. 2007, 15, 6574–6595. [Google Scholar] [CrossRef] [PubMed]
- Monovich, L.; Mugrage, B.; Quadros, E. Optimization of halopemide for phospholipase D2 inhibition. Bioorg. Med. Chem. Lett. 2007, 17, 2310–2311. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Selvy, P.E.; Buck, J.R. Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness. Nat. Chem. Biol. 2009, 5, 108–117. [Google Scholar]
- Lewis, J.A.; Scott, S.A.; Lavieri, R. Design and synthesis of isoform-selective phospholipase D (PLD) inhibitors. Part I: Impact of alternative halogenated privilegedstructures for PLD1 specificity. Bioorg. Med. Chem. Lett. 2009, 19, 1916–1920. [Google Scholar] [PubMed]
- Dwyer, M.; Keertikar, P.K.; Paruch, K. Discovery of pyrazolo[1,5-a]pyrimidine-based Piminhibitors: A template-based approach. Bioorg. Med. Chem. Lett. 2013, 23, 6178–6182. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.C.; Armstrong, A.; Chapman, K.L. Prospective use of molecular field points in ligand-based virtual screening: Efficient identification of new reversible Cdc25 inhibitors. Med. Chem. Commun. 2013, 4, 1148–1155. [Google Scholar] [CrossRef]
- Furber, M.; Alcaraz, L.; Luckhurst, C. Discovery and evolution of phenoxypiperidinehydroxyamide dual CCR3/H1 antagonists. Part I. Bioorg. Med. Chem. Lett. 2012, 22, 7702–7706. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, T.; Woods, K.W. Benzimidazolones and indoles as non-thiol farnesyltransferase inhibitors based on tipifarnib scaffold: Synthesis and activity. Bioorg. Med. Chem. Lett. 2005, 15, 2918–2922. [Google Scholar] [CrossRef] [PubMed]
- Steve, Y.; Cho, E.; Cynthia, M. Plasma and cerebrospinal fluid pharmacokinetics of intravenously administered ABT-751 in non-human primates. Cancer Chemother. Pharmacol. 2007, 4, 563–567. [Google Scholar]
- Sample Availability: Samples of all the compounds are available from the authors.




| No. | Compound | MDA-MB-48 | A549 | HCC1937 | Hek293 |
|---|---|---|---|---|---|
| 1 | 5a | 9 ± 0.67 | 5.2 ± 0.35 | 7.8 ± 0.49 | >10,000 |
| 2 | 5b | 6.3 ± 0.45 | 3.1 ± 0.17 | 2.6 ± 0.21 | 292.6 |
| 3 | 5c | >200 | 88 ± 6.1 | 105 ± 8.9 | 248.2 |
| 4 | 5d | 73 ± 6.9 | 77 ± 6.8 | 85 ± 7.1 | 756.4 |
| 5 | 5e | >200 | >200 | 127 ± 9.9 | >10,000 |
| 6 | 5f | 82 ± 6.1 | >200 | >200 | >10,000 |
| 7 | 5g | >200 | >200 | 152 ± 9.8 | 2000.9 |
| 8 | 5h | >200 | >200 | >200 | 369.7 |
| 9 | 5i | >200 | >200 | >200 | 708.9 |
| 10 | 5j | >200 | >200 | >200 | 665.9 |
| 11 | 5k | 135 ± 9.7 | 116 ± 7.6 | >200 | 383.6 |
| 12 | 5l | 22 ± 1.6 | 15 ± 1.1 | 18 ± 1.2 | 6312.3 |
| 13 | 5m | >200 | >200 | >200 | 447.6 |
| 14 | 5n | 12 ± 1.02 | 16 ± 0.9 | 22 ± 1.3 | >10,000 |
| 15 | 5o | 15 ± 0.93 | 9.7 ± 0.88 | 11 ± 0.71 | >10,000 |
| Doxorubicin | 2.7 ± 0.19 | 2.5 ± 0.13 | 4.8 ± 0.22 | 12.2 | |
| Pharmacophores that Fit Each Molecule | ||||||
|---|---|---|---|---|---|---|
| Name | Pharmacophore | Fit Value | Class | Subclass | Family | Acronym |
| 5b | 1e9h-inr-2.50-h-1 | 0.036708 | Enzymes | EC2. -(transferases) | Kinases (serine threonine) | CDK2 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, G.; Tong, R.; Li, J.; Bai, L.; Ouyang, L.; Duan, X.; Li, F.; He, P.; Shi, J.; He, Y. Synthesis and Antitumor Evaluation of Novel 5-Hydrosulfonyl-1H-benzo[d]imidazol-2(3H)-one Derivatives. Molecules 2016, 21, 516. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21040516
Ouyang G, Tong R, Li J, Bai L, Ouyang L, Duan X, Li F, He P, Shi J, He Y. Synthesis and Antitumor Evaluation of Novel 5-Hydrosulfonyl-1H-benzo[d]imidazol-2(3H)-one Derivatives. Molecules. 2016; 21(4):516. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21040516
Chicago/Turabian StyleOuyang, Guang, Rongsheng Tong, Jinqi Li, Lan Bai, Liang Ouyang, Xingmei Duan, Fengqiong Li, Pin He, Jianyou Shi, and Yuxin He. 2016. "Synthesis and Antitumor Evaluation of Novel 5-Hydrosulfonyl-1H-benzo[d]imidazol-2(3H)-one Derivatives" Molecules 21, no. 4: 516. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21040516