9,10-Dihydrophenanthrene with Two Spiro(dibenzocycloheptatriene) Units: A Highly Strained Caged Hydrocarbon Exhibiting Reversible Electrochromic Behavior
Abstract
:1. Introduction
2. Results and Discussion
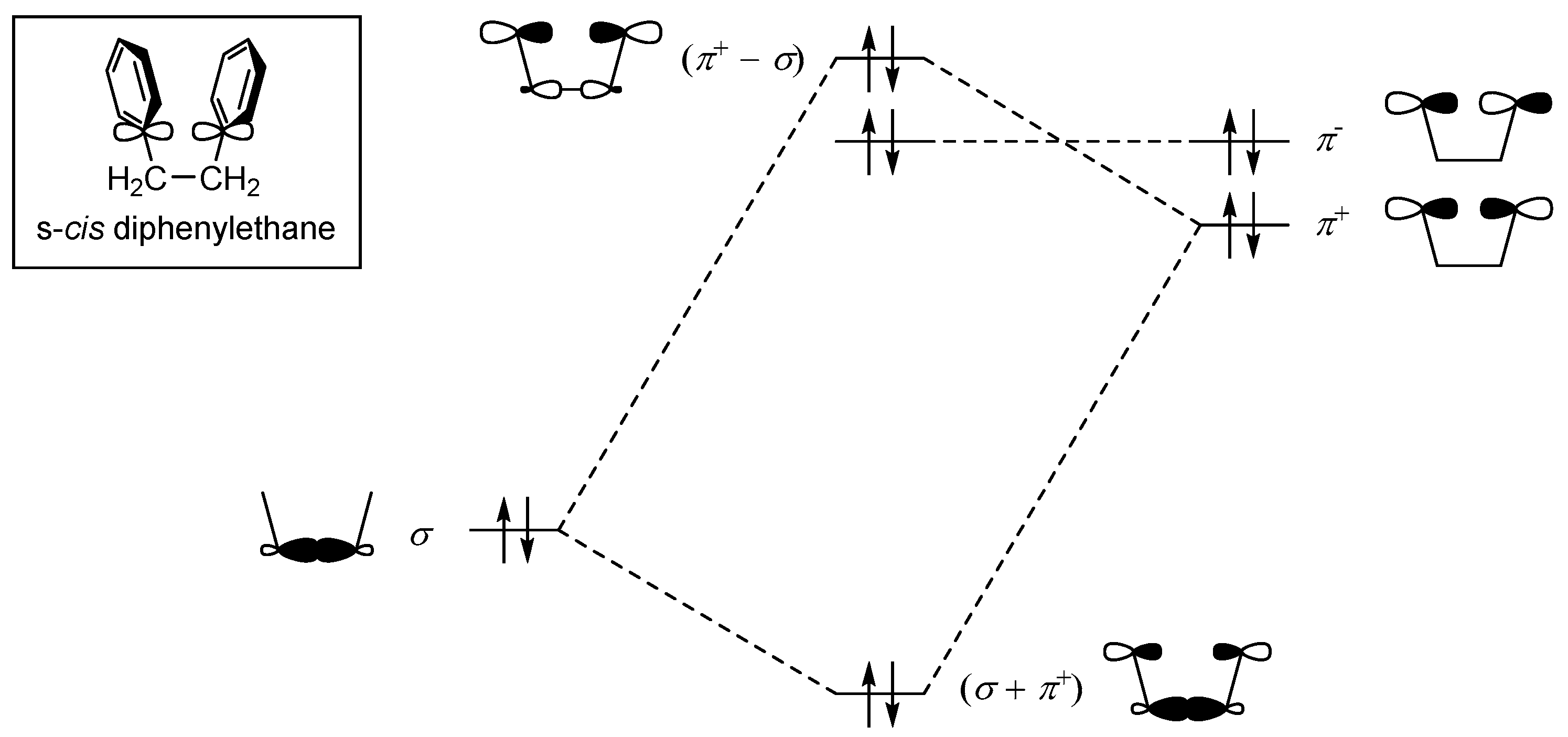
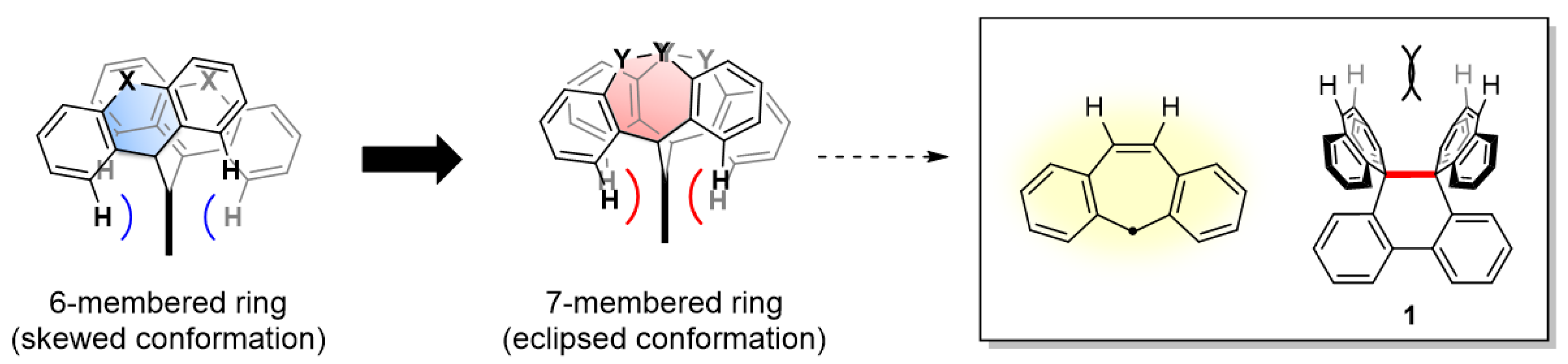
2.1. Design and Theoretical Study
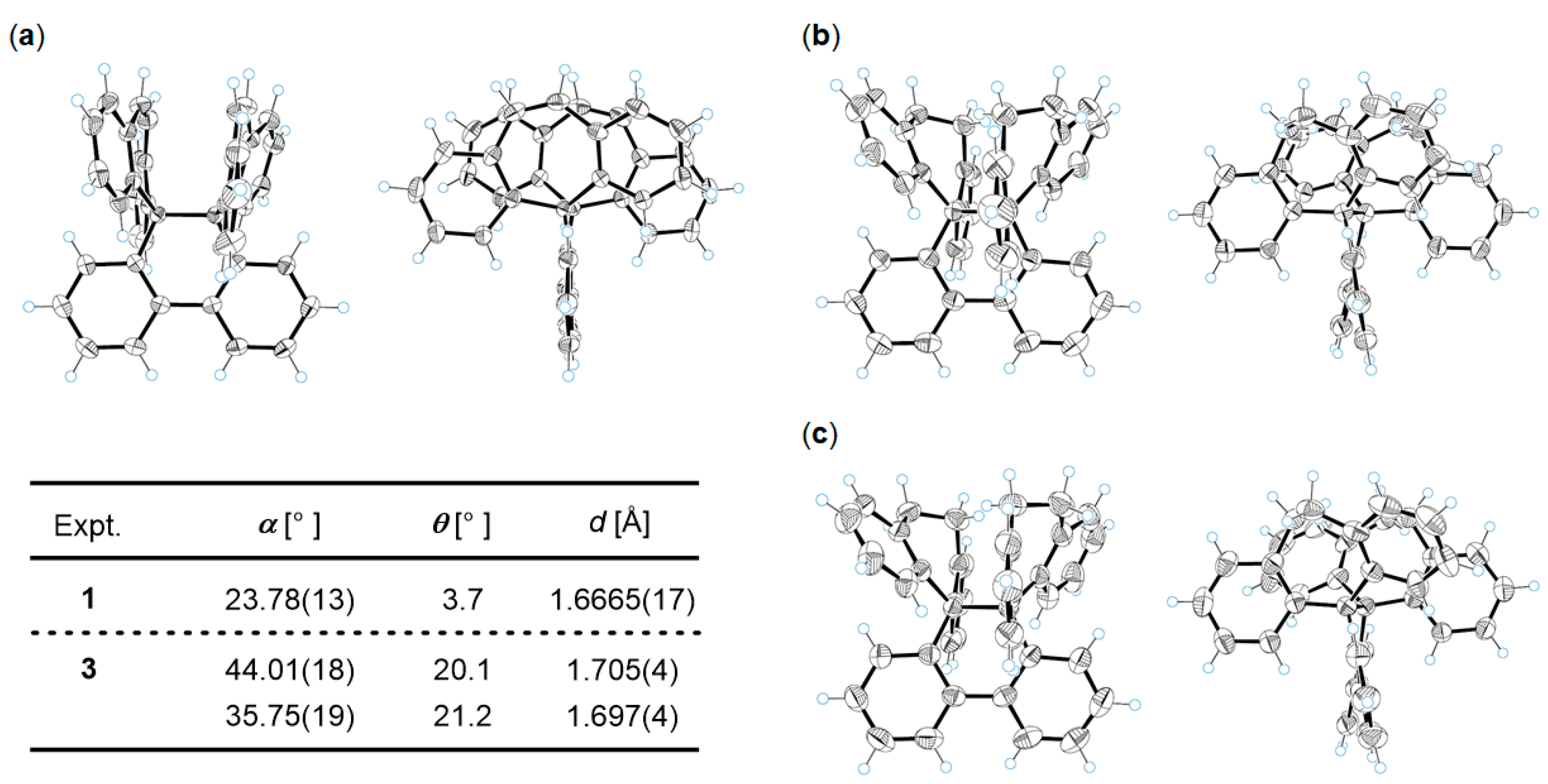
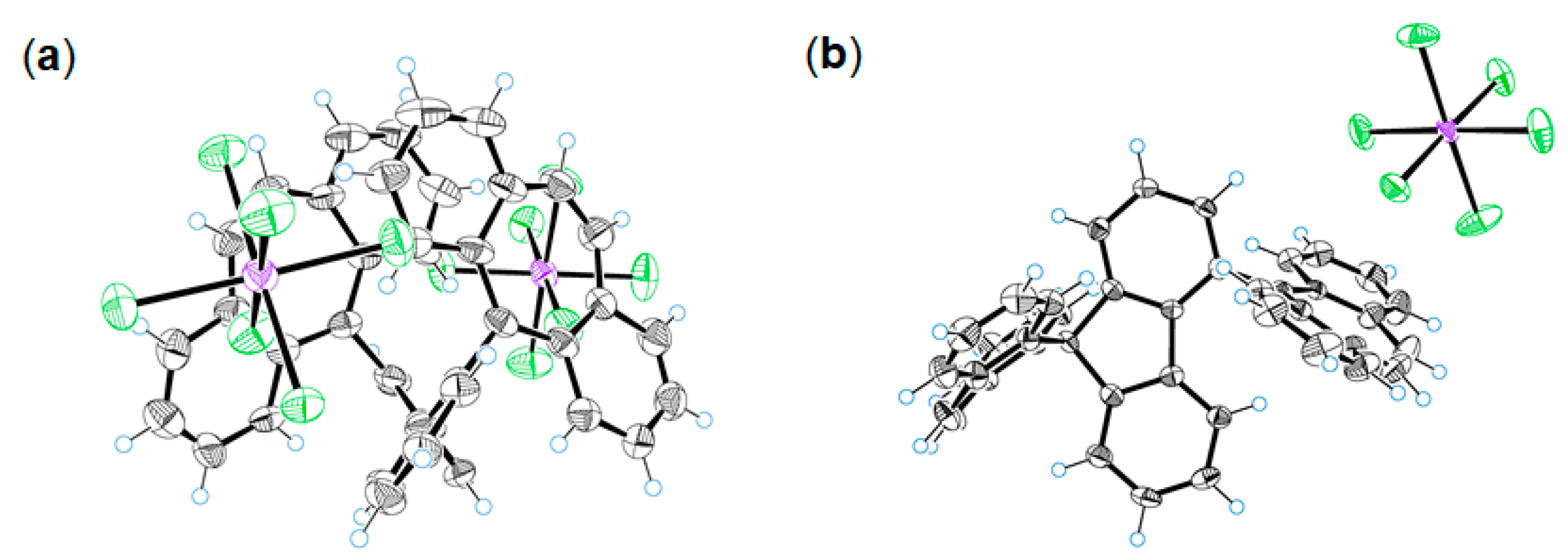
2.2. Preparation and X-ray Analysis
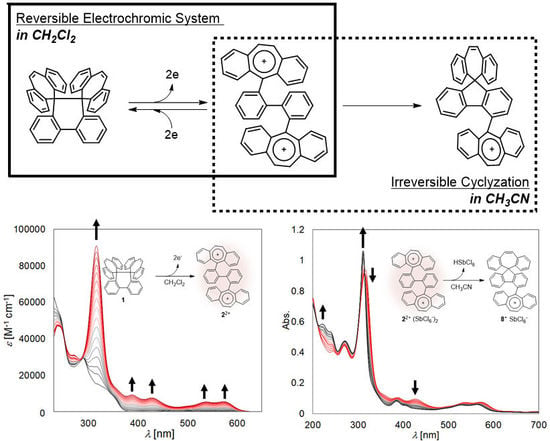
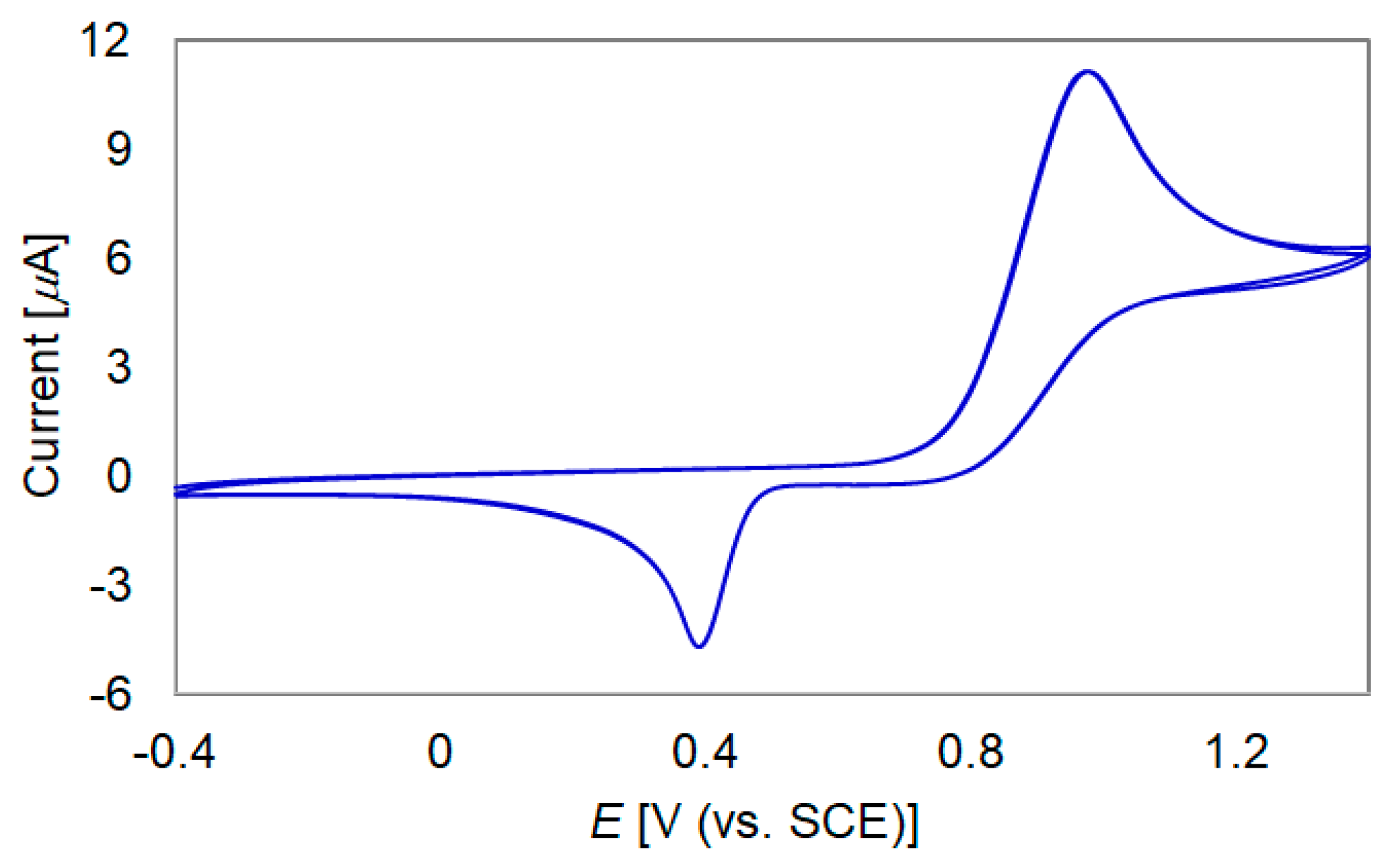
2.3. Redox Behavior
2.4. Electrochromic Behavior
3. Experimental
3.1. General Methods
3.2. Synthetic Procedures
3.2.1. Dispiro(dibenzo[a,d]cycloheptatriene-5,9′-phenanthrene-10′,5″-dibenzo[a,d]cycloheptatriene) 1
3.2.2. 5,5′-([1,1′-Biphenyl]-2,2′-diyl)bis(5H-dibenzo[a,d]cycloheptatrien-5-ylium) bis(tetrafluoroborate) 22+(BF4−)2
3.2.3. 5,5′-(1,1′-Biphenyl-2,2′-diyl)bis(5H-dibenzo[a,d]cycloheptatrien-5-ylium) bis(hexachloroantimonate) 22+(SbCl6−)2
3.2.4. Dispiro(dibenzo[a,d]cycloheptadiene-5,9′-phenanthrene-10′,5″-dibenzo[a,d]cycloheptadiene) 3
3.2.5. 2,2′-Bis(5-hydroxydibenzo[a,d]cycloheptatriene-5-yl)biphenyl 4 [37]
3.2.6. 2,2′-Bis(5-hydroxydibenzo[a,d]cycloheptadiene-5-yl)biphenyl 5 [37]
3.2.7. 5-(Spiro[dibenzo[a,d]cycloheptatriene-5,9′-fluoren]-4’-yl)-5H-dibenzo[a,d]cycloheptatriene-5-ylium hexachloroantimonate 8+(SbCl6−)
3.3. Crystal Data
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, Y.-T.; Siegel, J.S. Aromatic molecular-bowl hydrocarbons: synthetic derivatives, their structures, and physical properties. Chem. Rev. 2006, 106, 4843–4867. [Google Scholar] [CrossRef] [PubMed]
- Sygula, A.; Rabideau, P.W. Synthesis and chemistry of polycyclic aromatic hydrocarbons with curved Surfaces: Buckybowls. In Carbon-Rich Compounds; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2006; pp. 529–565. ISBN 9783527607990. [Google Scholar]
- Dodziuk, H. Strained Hydrocarbons; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2009; ISBN 9783527627134. [Google Scholar]
- Rieger, R.; Müllen, K. Forever young: Polycyclic aromatic hydrocarbons as model cases for structural and optical studies. J. Phys. Org. Chem. 2010, 23, 315–325. [Google Scholar] [CrossRef]
- Lu, J.; Ho, D.M.; Vogelaar, N.J.; Kraml, C.M.; Pascal, R.A. A Pentacene with a 144° Twist. J. Am. Chem. Soc. 2004, 126, 11168–11169. [Google Scholar] [CrossRef] [PubMed]
- Pascal, R.A. Twisted acenes. Chem. Rev. 2006, 106, 4809–4819. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.-N.; Kuo, M.-Y.; Wu, Y.-T. Synthesis, structural analysis, and properties of [8] circulenes. Angew. Chem. Int. Ed. 2013, 52, 7791–7794. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Suzuki, T. Tetrabenzo[8] circulene: Aromatic saddles from negatively curved graphene. J. Am. Chem. Soc. 2013, 135, 14074–14077. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, H.; Asada, T.; Kamikawa, K. Synthesis of a double helicene by a palladium-catalyzed cross-coupling reaction: Structure and physical properties. Chemistry 2015, 21, 6523–6527. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Segawa, Y.; Itami, K. Synthesis, structures, and properties of π-extended double helicene: A combination of planar and nonplanar π-systems. J. Am. Chem. Soc. 2015, 137, 7763–7768. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Segawa, Y.; Itami, K. Synthesis and structural features of quadruple helicenes: highly distorted π systems enabled by accumulation of helical repulsions. J. Am. Chem. Soc. 2016, 138, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Li, H.; Shan, B.; Liu, Z.; Miao, Q. Synthesis, structure, and properties of tetrabenzo[7] circulene. Org. Lett. 2017, 19, 2246–2249. [Google Scholar] [CrossRef] [PubMed]
- Kammermeier, S.; Jones, P.G.; Herges, R. [2+2] Cycloaddition products of tetradehydrodianthracene: experimental and theoretical proof of extraordinary long C–C single bonds. Angew. Chem. Int. Ed. Engl. 1997, 36, 1757–1760. [Google Scholar] [CrossRef]
- Tanaka, K.; Takamoto, N.; Tezuka, Y.; Kato, M.; Toda, F. Preparation and structural study of naphtho- and anthrocyclobutene derivatives which have extremely long C–C bonds. Tetrahedron 2001, 57, 3761–3767. [Google Scholar] [CrossRef]
- Kawai, H.; Takeda, T.; Fujiwara, K.; Inabe, T.; Suzuki, T. Exceptionally large difference in bond length among conformational isomorphs of a hexaphenylethane derivative with a dispiropyracene skeleton. Cryst. Growth Des. 2005, 5, 2256–2260. [Google Scholar] [CrossRef]
- Kawai, H.; Takeda, T.; Fujiwara, K.; Wakeshima, M.; Hinatsu, Y.; Suzuki, T. Ultralong carbon-carbon bonds in dispirobis(10-methylacridan) derivatives with an acenaphthene, pyracene, or dihydropyracylene skeleton. Chemistry 2008, 14, 5780–5793. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Kawai, H.; Herges, R.; Mucke, E.; Sawai, Y.; Murakoshi, K.; Fujiwara, K.; Suzuki, T. Negligible diradical character for the ultralong C–C bond in 1,1,2,2-tetraarylpyracene derivatives at room temperature. Tetrahedron Lett. 2009, 50, 3693–3697. [Google Scholar] [CrossRef]
- Takeda, T.; Uchimura, Y.; Kawai, H.; Katoono, R.; Fujiwara, K.; Suzuki, T. Hexaphenylethanes with an Ultralong C–C Bond: Expandability of the C–C Bond in Highly Strained Tetraarylpyracenes. Chem. Lett. 2013, 42, 954–962. [Google Scholar] [CrossRef]
- Suzuki, T.; Uchimura, Y.; Nagasawa, F.; Takeda, T.; Kawai, H.; Katoono, R.; Fujiwara, K.; Murakoshi, K.; Fukushima, T.; Nagaki, A.; et al. Expandability of ultralong C–C Bonds: Largely different C1–C2 bond lengths determined by low-temperature X-ray structural analyses on pseudopolymorphs of 1,1-bis(4-fluorophenyl)-2,2-bis(4-methoxyphenyl)pyracene. Chem. Lett. 2014, 43, 86–88. [Google Scholar] [CrossRef]
- Nishiuchi, T.; Uno, S.; Hirao, Y.; Kubo, T. Intramolecular interaction, photoisomerization, and mechanical C–C bond dissociation of 1,2-di(9-anthryl)benzene and its photoisomer: A fundamental moiety of anthracene-based π-cluster molecules. J. Org. Chem. 2016, 81, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Hounshell, W.D.; Dougherty, D.A.; Hummel, J.P.; Mislow, K. Structure of hexaphenylethane and congeners as determined by empirical force field calculations. J. Am. Chem. Soc. 1977, 99, 1916–1924. [Google Scholar] [CrossRef]
- Wittig, G.; Petri, H. Über das 9, 10-Tetraphenyl-dihydrophenanthren und 4,5-Dimethoxy-9, 10-tetraphenyl-dihydrophenanthren. (V. Mitt. über Ringschluß und Radikalbildung. Eur. J. Org. Chem. 1933, 505, 17–41. [Google Scholar] [CrossRef]
- Suzuki, T.; Takeda, T.; Kawai, H.; Fujiwara, K. Ultralong C-C bonds in hexaphenylethane derivatives. Pure Appl. Chem. 2008, 80, 547–553. [Google Scholar] [CrossRef]
- Wittig, G.; Schoch, W. Propellane des Dibenzo[g.p]chrysen-Systems. Eur. J. Org. Chem. 1971, 749, 38–48. [Google Scholar] [CrossRef]
- Debroy, P.; Lindeman, S.V.; Rathore, R. Hexabenzo[4.4.4]propellane: A helical molecular platform for the construction of electroactive materials. Org. Lett. 2007, 9, 4091–4094. [Google Scholar] [CrossRef] [PubMed]
- Dyker, G.; Körning, J.; Jones, P.G.; Bubenitschek, P. Palladium-catalyzed arylation of tetrasubstituted double bonds: A simple synthesis of annelated propellanes. Angew. Chem. Int. Ed. Engl. 1993, 32, 1733–1735. [Google Scholar] [CrossRef]
- Suzuki, T.; Nishida, J.; Tsuji, T. Hexaphenylethane derivatives exhibiting novel electrochromic behavior. Angew. Chem. Int. Ed. Engl. 1997, 36, 1329–1331. [Google Scholar] [CrossRef]
- Suzuki, T.; Nishida, J.; Tsuji, T. A new type of tricolor electrochromic system based on the dynamic redox properties of hexaarylethane derivatives. Chem. Commun. 1998, 2193–2194. [Google Scholar] [CrossRef]
- Suzuki, T.; Ono, K.; Nishida, J.; Takahashi, H.; Tsuji, T. Preparation and molecular structures of 9,10-dihydrophenanthrenes: Substituent effects on the long bond length. J. Org. Chem. 2000, 65, 4944–4948. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Migita, A.; Higuchi, H.; Kawai, H.; Fujiwara, K.; Tsuji, T. A novel redox switch for fluorescence: Drastic UV–vis and fluorescence spectral changes upon electrolysis of a hexaphenylethane derivative of 10,10′-dimethylbiacridan. Tetrahedron Lett. 2003, 44, 6837–6840. [Google Scholar] [CrossRef]
- Suzuki, T.; Ohta, K.; Nehira, T.; Higuchi, H.; Ohta, E.; Kawai, H.; Fujiwara, K. Unprecedented four-way-output molecular response system based on biphenyl-2,2′-diyldiacridiniums: Induction of axial chirality through intramolecular hydrogen bonds between chiral amide groups. Tetrahedron Lett. 2008, 49, 772–776. [Google Scholar] [CrossRef]
- Suzuki, T.; Tamaoki, H.; Nishida, J.; Higuchi, H.; Iwai, T.; Ishigaki, Y.; Hanada, K.; Katoono, R.; Kawai, H.; Fujiwara, K.; et al. Redox-mediated reversible σ-bond formation/cleavage. In Organic Redox Systems; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 13–37. ISBN 9781118858981. [Google Scholar]
- Kahr, B.; Van Engen, D.; Mislow, K. Length of the ethane bond in hexaphenylethane and its derivatives. J. Am. Chem. Soc. 1986, 108, 8305–8307. [Google Scholar] [CrossRef]
- Atoji, M.; Oda, T.; Watanabé, T. On the crystal structure of cubic hexachloroethane. Acta Crystallogr. 1953, 6, 868. [Google Scholar] [CrossRef]
- Hoffmann, R. Interaction of orbitals through space and through bonds. Acc. Chem. Res. 1971, 4, 1–9. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01, Gaussian, Inc.: Wallingford, CT, USA, 2013.
- Weber, E.; Wierig, A.; Skobridis, K. Crystalline diol hosts featuring a bulky biphenyl framework—Host Synthesis and Formation of Inclusion Compounds. Adv. Synth. Catal. 1996, 338, 553–557. [Google Scholar] [CrossRef]
- Saitoh, T.; Yoshida, S.; Ichikawa, J. 1,8-Bis(diphenylmethylium)naphthalenediyl dication as an organic oxidant: Synthesis of benzidines via self-coupling of N,N-dialkylanilines. Org. Lett. 2004, 6, 4563–4565. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Takeda, T.; Kawai, H.; Katoono, R.; Fujiwara, K.; Suzuki, T. Geometrical remote steric effects in 4,5-disubstituted-9,10-dihydrophenanthrenes: expansion of prestrained C9–C10 bond in di(spiroacridan) derivatives. Chem. Lett. 2013, 42, 1194–1196. [Google Scholar] [CrossRef]
- Ito, S.; Morita, N. Creation of stabilized electrochromic materials by taking advantage of azulene skeletons. Eur. J. Org. Chem. 2009, 2009, 4567–4579. [Google Scholar] [CrossRef]
- Suzuki, T.; Iwai, T.; Ohta, E.; Kawai, H.; Fujiwara, K. Electrochiroptical systems based on biphenyl-2,2′-diyl-type dicationic dyes: Strong chiroptical signals through the transmission of point chirality to axial chirality. Tetrahedron Lett. 2007, 48, 3599–3603. [Google Scholar] [CrossRef]
- Suzuki, T.; Ishigaki, Y.; Iwai, T.; Kawai, H.; Fujiwara, K.; Ikeda, H.; Kano, Y.; Mizuno, K. Multi-input/multi-output molecular response system based on the dynamic redox behavior of 3,3,4,4-tetraaryldihydro[5]helicene derivatives: reversible formation/destruction of chiral fluorophore and modulation of chiroptical properties by solvent polarity. Chemistry 2009, 15, 9434–9441. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Kinson, P.L.; Maier, C.A.; Paul, I.C. Structure of 4,8-dihydrodibenzo[cd,gh] pentalene. J. Am. Chem. Soc. 1971, 93, 7275–7281. [Google Scholar] [CrossRef]
- Neugebauer, W.; Kos, A.J.; von Ragué Schleyer, P. Regioselektive dimetallierung von aromaten. Bequemer zugang zu 2,2′-disubstituierten biphenylderivaten. J. Organomet. Chem. 1982, 228, 107–118. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–8 are available from the authors. |






© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishigaki, Y.; Hayashi, Y.; Sugawara, K.; Shimajiri, T.; Nojo, W.; Katoono, R.; Suzuki, T. 9,10-Dihydrophenanthrene with Two Spiro(dibenzocycloheptatriene) Units: A Highly Strained Caged Hydrocarbon Exhibiting Reversible Electrochromic Behavior. Molecules 2017, 22, 1900. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22111900
Ishigaki Y, Hayashi Y, Sugawara K, Shimajiri T, Nojo W, Katoono R, Suzuki T. 9,10-Dihydrophenanthrene with Two Spiro(dibenzocycloheptatriene) Units: A Highly Strained Caged Hydrocarbon Exhibiting Reversible Electrochromic Behavior. Molecules. 2017; 22(11):1900. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22111900
Chicago/Turabian StyleIshigaki, Yusuke, Yuki Hayashi, Kazuma Sugawara, Takuya Shimajiri, Wataru Nojo, Ryo Katoono, and Takanori Suzuki. 2017. "9,10-Dihydrophenanthrene with Two Spiro(dibenzocycloheptatriene) Units: A Highly Strained Caged Hydrocarbon Exhibiting Reversible Electrochromic Behavior" Molecules 22, no. 11: 1900. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22111900