Chitin and Cellulose Processing in Low-Temperature Electron Beam Plasma †
Abstract
:1. Introduction
- To study the EBP-processing of chitin and cellulose and to reveal the time dependence for the EBP-stimulated formation of water-soluble low molecular weight products (LMWP).
- To characterize the chemical structure and molecular mass of the produced LMWP.
- To suggest possible mechanisms of EBP action on polysaccharides.
2. Results
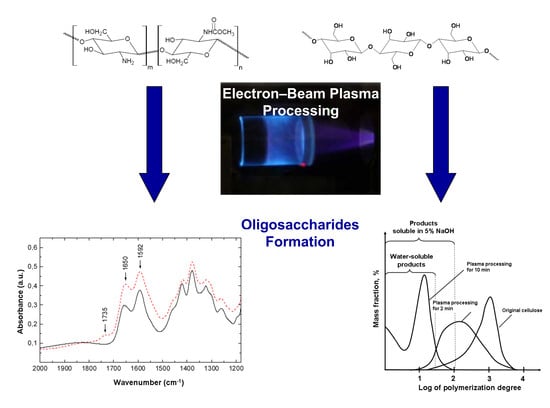
2.1. The EBPProcessing of Polysaccharides
2.2. The Characterization of the EBP-Treated Polysaccharides
2.2.1. Molecular Mass Characterization
2.2.2. Main Features of the Polysaccharides EBP-Processing
- At optimized treatment conditions (plasma generating media pressure, electron beam characteristics, the mixing device design)only 2 min were required to obtain 85% yield of low-molecular weight COS from original chitin powder [17]. The maximal yield of cellulose water-soluble LMWP (77.4%) was obtained after 10 min (Table 1).
- The threshold relationship between LMWP yield and the duration of the EBP-treatment. At optimized treatment conditions, chitin and cellulose destruction stopped when the processing time reached 7 and 15 min, respectively.
2.2.3. Chemical Structure Characterization
DA = 50.9 mol %,
DD = 49.1 mol %
3. Discussion
3.1. Possible Mechanisms of the EBP-Action on Polysaccharides
- Chemically active heavy particles of the EBP: excited molecules and atoms, ions, radicals (active oxygen species, for example);
- The fast electrons of the partially degraded EB that bombard the polysaccharide material;
- The secondary electrons of moderate energies (up to 0.01–1 keV) produced in the EBP due to ionization of the molecules of the plasma generating media;
- The EBP-radiation, especially UV one and X-ray (bremsstrahlung);
- Possible heating by direct electron bombardment and due to heat transfer between the plasma cloud and the sample.
3.2. The Comparison of the EBP-Stimulated Polysaccharides Destruction with Respect to Acid-Catalyzed Hydrolysis
4. Materials and Methods
5. Conclusions
- The possibility of the EBP-stimulated degradation of chitin and cellulose and formation of the water-soluble oligosaccharides was proved experimentally.
- Chitooligosaccharides with weight-average molecular mass 1000–2000 Da and polydispersion index 2.2 and cellulose oligosaccharides with polymerization degrees 3–10 were formed due to the destruction of β-1,4-glycosidic bonds during the EBP-treatment. The EBP-processing resulted in 25–40% deacetylation of original chitin and partial oxidation of the produced oligosaccharides.
- At optimized treatment conditions (plasma generating media pressure, electron beam characteristics, design of the mixing device) 85% yield of low-molecular weight COS and 77.4% yield of cellulose oligosaccharides were obtained in only 2 and 10 min, respectively.
- Both the fast electrons and the active oxygen particles produced in the EBP are responsible for the destruction of β-1,4-glycosidic bonds in the original polysaccharides. By proper selection of treatment conditions, the polysaccharide damage due to high-energy electrons can be minimized and the plasma chemical processes predominate.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Schnepp, Z. Biopolymers as a flexible resource for nanochemistry. Angew. Chem. Int. Ed. 2013, 52, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Paulova, L.; Patakova, P.; Branska, B.; Rychtera, M.; Melzoch, K. Lignocellulosic ethanol: Technology design and its impact on process efficiency. Biotechnol. Adv. 2014, 33, 1091–1107. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.D. Potential aspects of chitosan as pharmaceutical excipient. Acta Pol. Pharm. 2011, 68, 619–622. [Google Scholar] [PubMed]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Zhang, Z.H.; Jin, X.; Jiang, Y.R.; Jia, X.B. Enhanced dissolution and oral bioavailability of tanshinone IIA base by solid dispersion system with low-molecular-weight chitosan. J. Pharm. Pharmacol. 2013, 65, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Puras, G.; Zarate, J.; Aceves, M.; Murua, A.; Diaz, A.R.; Aviles-Triguero, M.; Fernandez, E.; Pedraz, J.L. Low molecular weight oligochitosans for non-viral retinal gene therapy. Eur. J. Pharm. Biopharm. 2013, 83, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Silva-Dias, A.; Palmeira-de-Oliveira, A.; Miranda, I.M.; Branco, J.; Cobrado, L.; Monteiro-Soares, M.; Queiroz, J.A.; Pina-Vaz, C.; Rodrigues, A.G. Anti-biofilm activity of low-molecular weight chitosan hydrogel against Candida species. Med. Microbiol. Immun. 2014, 203, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.G. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef] [Green Version]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Goycoolea, F.; Agullo, E.; Mato, R. Fuentes y procesos de obtencion. In Quitina y Quitosano: Obtencion, Caracterization y Aplicaciones; de Abram, A.P., Ed.; Pontificia Universidad Catolica del Peru, Fondo Editoral: Lima, Peru, 2004; pp. 105–156. ISBN 9972-42-659-9. [Google Scholar]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Gupta, R.; Demirbas, A. Renewable energy sources. In Gasoline, Diesel, and Ethanol Biofuels from Grasses and Plants, 1st ed.; Gupta, R., Demirbas, A., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 41–55. ISBN 978-0-521-76399-8. [Google Scholar]
- Verardi, A.; de Bari, I.; Ricca, E.; Calabro, V. Hydrolisys of ligninocellulosic biomass: Current status of process and technologies and future perspectives. In Bioethanol; Lima, M.A., Natalense, A.P.P., Eds.; Intech: Rijeka, Croatia, 2012; pp. 95–122. ISBN 978-953-51-0008-9. [Google Scholar]
- Yang, B.; Dai, Z.; Ding, S.Y.; Wyman, C.E. Enzymatic hydrolysis of cellulosic biomass. Biofuels 2011, 2, 421–450. [Google Scholar] [CrossRef]
- Vasilieva, T.; Lopatin, S.; Varlamov, V.; Miasnikov, V.; Hein, A.M.; Vasiliev, M. Hydrolisys of chitin and chitosan in low temperature electron-beam plasma. Pure Appl. Chem. 2016, 88, 873–879. [Google Scholar] [CrossRef]
- Vasilieva, T. A beam-plasma source for protein modification technology. IEEE Trans. Plasma Sci. 2010, 38, 1903–1907. [Google Scholar] [CrossRef]
- Vasiliev, M.; Vasilieva, T. Materials production with beam plasmas. In Encyclopedia of Plasma Technology; Shohet, J.L., Ed.; Taylor & Francis Group: New York, NY, USA, 2016; pp. 52–166. ISBN 978-1-4665-0059-4. [Google Scholar]
- Balau, L.; Lisa, G.; Popa, M.I.; Tura, V.; Melnig, V. Physico-chemical properties of chitosan films. Cent. Eur. J. Chem. 2004, 2, 638–647. [Google Scholar] [CrossRef]
- Glavan, N.; Krstulovic, N.; Cutic, N.; Milosevic, S.; Cvelbar, U.; Vesel, A.; Drenik, A.; Mozetic, M. Optical emission spectroscopy characterization of low pressure plasma created in water vapor. Vacuumist 2005, 25, 29–33. [Google Scholar]
- Fridman, A. Plasma Chemistry; Cambridge University Press: New York, NY, USA, 2008; pp. 318–333. ISBN 978-0-521-84735-3. [Google Scholar]
- Ehrlich, H.; Maldonado, M.; Spindler, K.-D.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. PartI. Verongidae (Demospongia: Porifera). J. Exp. Zool. 2007, 308, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Czerwicka, M.; Kaczynski, Z.; Bychowska, A.; Brzozowski, K.; Thoming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 567–1636. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wang, Z.; Zhao, H.; Tian, S. Plasma depolymerization of chitosan in the presence of hydrogen peroxide. Int. J. Mol. Sci. 2012, 13, 7788–7797. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Xiao, R.; Gu, S.; Zhang, H. The overview of thermal decomposition of cellulose in lignocellulosic biomass. In Cellulose—Biomass Conversion; van de Ven, T., Kadla, J., Eds.; Intech: Rijeka, Croatia, 2013; pp. 193–226. ISBN 978-953-51-1172-6. [Google Scholar]
- Vasilieva, T.; Lysenko, S. Factors responsible for biomaterials modification in the electron-beam plasma. J. Phys. Conf. Ser. 2007, 63, 012033. [Google Scholar] [CrossRef]
- Vasil’eva, T.M.; Chukhchin, D.G. The effect of beam-plasma modification of fibrin-monomer on its biological properties. High Energy Chem. 2008, 42, 404–407. [Google Scholar] [CrossRef]
- Gilbert, B.C.; King, D.M.; Thomas, B. Radical reactions of carbohydrates. Part 2. An electron spin resonance study of the oxidation of d-glucose and related compounds with the hydroxyl radical. J. Chem. Soc. Perkin Trans. 1981, 2, 1186–1199. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Direct detection and identification of radicals generated during the hydroxyl radical-induced degradation of hyaluronic acid and related materials. Free Radic. Biol. Med. 1996, 21, 275–290. [Google Scholar] [CrossRef]
- Duan, J.; Kasper, D.L. Oxidative depolymerization of polysaccharides by reactive oxygen/nitrogen species. Glycobiology 2011, 21, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.L.B.; Tai, M.C.; Cheng, F.H. Kinetics and products of the degradation of chitosan by hydrogen peroxide. J. Agric. Food Chem. 2001, 49, 4845–4851. [Google Scholar] [CrossRef] [PubMed]
- Rees, M.D.; Kennett, E.C.; Whitelock, J.M.; Davies, M.J. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radic. Biol. Med. 2008, 44, 1973–2001. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Priyadarsini, K.I.; Mohan, H. Pulse radiolysis studies on reaction of OH radical with N-acetyl methionine in aqueous solution. Res. Chem. Intermed. 2005, 31, 625–632. [Google Scholar] [CrossRef]
- Reeds, M.D.; Hawkins, C.L.; Davies, M.J. Hypochlorite and superoxide radicals can act synergistically to induce fragmentation of hyaluronan and chondroitin sulphates. Biochem. J. 2004, 381, 175–184. [Google Scholar]
- Stern, R.; Kogan, G.; Jedrzejas, M.J.; Soltes, L. The many ways to cleave hyaluronan. Biotechnol. Adv. 2007, 25, 537–557. [Google Scholar] [CrossRef] [PubMed]
- Van Deynse, A.; Morent, R.; de Geyter, N. Surface modification of polymers using atmospheric pressure cold plasma technology. In Polymer Science: Research Advances, Practical Applications and Educational Aspects; Méndez-Vilas, A., Solano, A., Eds.; Formatex Research Center: Badajoz, Spain, 2016; pp. 506–516. ISBN 978-84-942134-8-9. [Google Scholar]
- Encinas, N.; Pantoja, M.; Abenojar, J.; Martinez, M.A. Control of wettability of polymers by surface roughness modification. J. Adhes. Sci. Technol. 2010, 24, 1869–1883. [Google Scholar] [CrossRef]
- Geyter, N.; Dubruel, P.; Morent, R.; Leys, C. Plasma assisted surface modification of polymeric biomaterials. In Low Temperature Plasma Technology: Methods and Applications; Chu, P.K., Lu, X., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 401–418. ISBN 978-1-4665-0991-7. [Google Scholar]
- Rabinovich, M.L. Wood hydrolysis industry in the Soviet Union and Russia: Mini-review. Cellul. Chem. Technol. 2010, 44, 173–186. [Google Scholar]
- Uchida, Y.; Izume, M.; Ohtakara, A. Preparation of chitosan oligosaccharides with purified chitosanase and its applications. In Chitin and Chitosan; Break, G., Anthonsen, T., Sandford, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 373–382. ISBN 978-1-85166-395-8. [Google Scholar]
- Ogino, A.; Kral, M.; Yamashita, M.; Nagatsu, M. Effect of low-temperature surface-wave plasma treatment with various gases on surface modification of chitosan. Appl. Surf. Sci. 2008, 255, 2347–2352. [Google Scholar] [CrossRef]
- Pavlinak, D.; Svachova, V.; Vojtek, L.; Zarzycka, J.; Hyrsl, P.; Alberti, M.; Voojtova, L. Plasma-chemical modification of cellulose for biomedical applications. Open Chem. 2015, 13, 229–235. [Google Scholar]
- Pertile, R.A.N.; Andrade, F.K.; Gama, C.A.M., Jr. Surface modification of bacterial cellulose by nitrogen-containing plasma for improved interactions with cells. Carbohydr. Polym. 2010, 82, 692–698. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Text. Res. J. 1962, 29, 786–794. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |













| Time of EBP-Processing, min | Yield of Water-Soluble Products, % | Yield of Products, Soluble in 5% NaOH, % | Yield of Non-Soluble Residue 1, % | Polymerization Degree of Non-Soluble Residue |
|---|---|---|---|---|
| 0 | 1.8 | 6.8 | 93.2 | 960 |
| 2 | 22.7 | 45.3 | 54.7 | 460 |
| 10 | 77.4 | 100 | 0 | - |
| Criteria | Chemical Hydrolysis | EBP-Processing |
|---|---|---|
| Treatment time | Several hours or days | Minutes (τ = 2–10) |
| Number of stages | Multi-stage | Single-stage |
| Specificity and Efficiency | Destruction of β-1,4-glycosidic bonds; Destruction of amorphous parts of biopolymer predominates; Both oxidation changes of produced oligosaccharides and deep oxidation of original biopolymers are possible. | Destruction of β-1,4-glycosidic bounds; Destruction of both amorphous and crystalline parts of biopolymer occurs; Oxidation changes of oligosaccharides (formation of C=O and –COOH groups). |
| Chitin deacetylation | Deacetylated low molecular weight derivatives of chitin are produced. Reacetylation is needed | The DD of produced chitin oligosaccharides 25–40%. |
| Molecular mass of products | Cellulose fragments with polymerization degree 200 or monomers are produced [40] | LMWP from dimers to heptamers are produced. |
| LMWP yields | Low yields of LMWP and large amounts of monomeric units [40,41]. | Up to 80–85% |
| Ecological safety | Highly concentrated acidic or alkaline solutions are needed. Toxic wastes are produced. High energy consumption. | Environmentally friendly: hazardous byproducts and toxic wastes are not generated. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilieva, T.; Chuhchin, D.; Lopatin, S.; Varlamov, V.; Sigarev, A.; Vasiliev, M. Chitin and Cellulose Processing in Low-Temperature Electron Beam Plasma. Molecules 2017, 22, 1908. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22111908
Vasilieva T, Chuhchin D, Lopatin S, Varlamov V, Sigarev A, Vasiliev M. Chitin and Cellulose Processing in Low-Temperature Electron Beam Plasma. Molecules. 2017; 22(11):1908. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22111908
Chicago/Turabian StyleVasilieva, Tatiana, Dmitry Chuhchin, Sergey Lopatin, Valery Varlamov, Andrey Sigarev, and Michael Vasiliev. 2017. "Chitin and Cellulose Processing in Low-Temperature Electron Beam Plasma" Molecules 22, no. 11: 1908. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22111908