Mechanistic Explanation of the Weak Carbonic Anhydrase’s Esterase Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Enzyme-Substrate Complex
2.2. Hydrolysis of the Substrate
Restoration of the Catalyst
3. Computational Methods
4. Conclusions
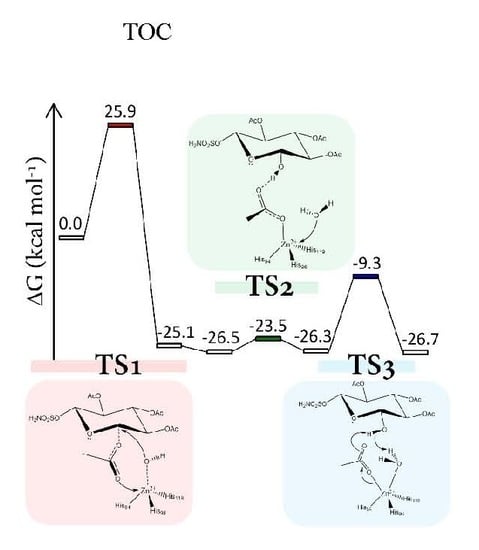
- The rate-limiting step of the process is a concerted event where the C-1 acyl group coordinates the zinc ion with the concomitant nucleophilic attack of the OH ion towards the anomeric carbon.
- The related activation energy is 25.9 kcal·mol−1 and can explain the weak esterase activity of carbonic anhydrase.
- The hydrolyzed product shows a consistent energy stabilization with respect to the ES complex, confirming that the fully-acetylated sugar-based sulfamates first bind as a substrate, and after act as an inhibitor.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lewis, J.C. Artificial Metalloenzymes and Metallopeptide Catalysts for Organic Synthesis. ACS Catal. 2013, 3, 2954–2975. [Google Scholar] [CrossRef]
- Farwell, C.C.; Zhang, R.K.; McIntosh, J.A.; Hyster, T.K. Enantioselective Enzyme-Catalyzed Aziridination Enabled by Active-Site Evolution of a Cytochrome P450. ACS Cent. Sci. 2015, 1, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Hyster, T.K.; Arnold, F.H. P450 BM3-Axial Mutations: A Gateway to Non-Natural Reactivity. Isr. J. Chem. 2015, 55, 14–20. [Google Scholar] [CrossRef]
- Hult, K.; Berglund, P. Enzyme promiscuity: Mechanism and applications. Trends. Biotechnol. 2007, 25, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Khersonsky, O.; Tawfik, D.S. Enzyme promiscuity: A mechanistic and evolutionary perspective. Annu. Rev. Biochem. 2010, 79, 471–505. [Google Scholar] [PubMed]
- Bornscheuer, U.T.; Kazlauskas, R.J. Catalytic promiscuity in biocatalysis: Using old enzymes to form new bonds and follow new pathways. Angew. Chem. Int. Ed. Engl. 2004, 43, 6032–6040. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskas, R.J. Enhancing catalytic promiscuity for biocatalysis. Curr. Opin. Chem. Biol. 2005, 9, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Nobeli, I.; Favia, A.D.; Thornton, J.M. Protein promiscuity and its implications for biotechnology. Nat. Biotechnol. 2009, 27, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Uda, N.R.; Seibert, V.; Stenner-Liewen, F.; Müller, P.; Herzig, P.; Gondi, G.; Zeidler, R.; Dijk, M.van; Zippelius, A.; Renner, C.; et al. Esterase activity of carbonic anhydrases serves as surrogate for selecting antibodies blocking hydratase activity. J. Enzyme Inhib. Med. Chem. 2015, 30, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Lindskog, S. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar] [CrossRef]
- Breton, S. The Cellular Physiology of Carbonic Anhydrases. J. Pancreas 2001, 2, 159–164. [Google Scholar]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Chiche, J.; Ilc, K.; Laferrière, J.; Trottier, E.; Dayan, F.; Mazure, N.M.; Brahimi-Horn, M.C.; Pouysségur, J. Hypoxia-Inducible Carbonic Anhydrase IX and XII Promote Tumor Cell Growth by Counteracting Acidosis through the Regulation of the Intracellular pH. Cancer Res. 2009, 69, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Swietach, P.; Wigfield, S.; Cobden, P.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growths. J. Biol. Chem. 2008, 283, 20473–20477. [Google Scholar] [CrossRef] [PubMed]
- Swietach, P.; Patiar, S.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. The Role of Carbonic Anhydrase 9 in Regulating Extracellular and Intracellular pH in Three-dimensional Tumor Cell Growths. J. Biol. Chem. 2009, 284, 20299–20310. [Google Scholar] [CrossRef] [PubMed]
- Svastová, E.; Hulíková, A.; Rafajová, M.; Zat’ovicová, M.; Gibadulinová, A.; Casini, A.; Cecchi, A.; Scozzafava, A.; Supuran, C.T.; Pastorek, J.; et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004, 577, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Babtie, A.; Tokuriki, N.; Hollfelder, F. What makes an enzyme promiscuous? Curr. Opin. Chem. Biol. 2010, 14, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Pocker, Y.; Stone, J.T. The Catalytic Versatility of Erythrocyte Carbonic Anhydrase. The Enzyme-Catalyzed Hydrolysis of p-Nitrophenyl Acetate. J. Am. Chem. Soc. 1965, 87, 5497–5498. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.M.; Tawfik, D.S. Directed evolution of the promiscuous esterase activity of carbonic anhydrase II. Biochemistry 2005, 44, 5444–5452. [Google Scholar] [CrossRef] [PubMed]
- Fierke, C.A.; Calderone, T.L.; Krebs, J.F. Functional consequences of engineering the hydrophobic pocket of carbonic anhydrase II. Biochemistry 1991, 30, 11054–11063. [Google Scholar] [CrossRef] [PubMed]
- Elleby, B.; Sjöblom, B.; Lindskog, S. Changing the efficiency and specificity of the esterase activity of human carbonic anhydrase II by site-specific mutagenesis. Eur. J. Biochem. 1999, 262, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.S.; Nair, S.K.; Christianson, D.W. Engineering the hydrophobic pocket of carbonic anhydrase II. Biochemistry 1991, 30, 11064–11072. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.S.; Kiefer, L.L.; Fierke, C.A.; Christianson, D.W. Engineering the zinc binding site of human carbonic anhydrase II: Structure of the His-94.fwdarw.Cys apoenzyme in a new crystalline form. Biochemistry 1993, 32, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Vu, H.; Wang, C.K.; Wolf, M.G.; Groenhof, G.; Innocenti, A.; Supuran, C.T.; Poulsen, S.-A. Promiscuity of carbonic anhydrase II. Unexpected ester hydrolysis of carbohydrate-based sulfamate inhibitors. J. Am. Chem. Soc. 2011, 133, 18452–18462. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.F.; Ippolito, J.A.; Christianson, D.W.; Fierke, C.A. Structural and functional importance of a conserved hydrogen bond network in human carbonic anhydrase II. J. Biol. Chem. 1993, 268, 27458–27466. [Google Scholar] [PubMed]
- Aharoni, A.; Gaidukov, L.; Khersonsky, O.; McQGould, S.; Roodveldt, C.; Tawfik, D.S. The ‘evolvability’ of promiscuous protein functions. Nat. Genet. 2005, 37, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Trajkovic, J.; Bornaghi, L.F.; Innocenti, A.; Vullo, D.; Supuran, C.T.; Poulsen, S.-A. Design, Synthesis, and Biological Evaluation of Novel Carbohydrate-Based Sulfamates as Carbonic Anhydrase Inhibitors. J. Med. Chem. 2011, 54, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Lavis, L.D. Bright ideas for chemical biology. ACS Chem. Biol. 2008, 3, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Piazzetta, P.; Marino, T.; Russo, N. Promiscuous Ability of Human Carbonic Anhydrase: QM and QM/MM Investigation of Carbon Dioxide and Carbodiimide Hydration. Inorg. Chem. 2014, 53, 3488–3493. [Google Scholar] [CrossRef] [PubMed]
- Piazzetta, P.; Marino, T.; Russo, N. Insight into the promiscuous activity of human carbonic anhydrase against the cyanic acid substrate from a combined QM and QM/MM investigation. Phys. Chem. Chem. Phys. 2014, 16, 16671–16676. [Google Scholar] [CrossRef] [PubMed]
- Piazzetta, P.; Marino, T.; Russo, N.; Salahub, D.R. The role of metal substitution in the promiscuity of natural and artificial carbonic anhydrases. Coord. Chem. Rev. 2017, 345, 73–85. [Google Scholar] [CrossRef]
- Gaspari, R.; Rechlin, C.; Heine, A.; Bottegoni, G.; Rocchia, W.; Schwarz, D.; Bomke, J.; Gerber, H.-D.; Klebe, G.; Cavalli, A.; et al. Kinetic and Structural Insights into the Mechanism of Binding of Sulfonamides to Human Carbonic Anhydrase by Computational and Experimental Studies. J. Med. Chem. 2016, 59, 4245–4256. [Google Scholar] [CrossRef] [PubMed]
- Dudev, T.; Lim, C. Tetrahedral vs Octahedral Zinc Complexes with Ligands of Biological Interest: A DFT/CDM Study. J. Am. Chem. Soc. 2000, 122, 11146–11153. [Google Scholar] [CrossRef]
- Miscione, G.P.; Stenta, M.; Spinelli, D.; Anders, E.; Bottoni, A. New computational evidence for the catalytic mechanism of carbonic anhydrase. Theor. Chem. Acc. 2007, 118, 193–201. [Google Scholar] [CrossRef]
- Höst, G.; Mårtensson, L.-G.; Jonsson, B.-H. Redesign of human carbonic anhydrase II for increased esterase activity and specificity towards esters with long acyl chains. Biochim. Biophys. Acta. 2006, 10, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Jencks, W.P. Binding energy, specificity, and enzymic catalysis: The Circe effect. Adv. Enzymol. Relat. Areas Mol. Biol. 1975, 43, 219–410. [Google Scholar] [PubMed]
- Åqvist, J.; Kazemi, M.; Isaken, G.V.; Bradsdal, B.O. Entropy and Enzyme Catalysis. Acc. Chem. Res. 2017, 50, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Himo, F.; Åqvist, J. Dispelling the effects of a sorceress in enzyme catalysis. Proc. Natl. Acad. Sci. USA 2016, 113, 2406–2411. [Google Scholar] [CrossRef] [PubMed]
- Piazzetta, P.; Marino, T.; Russo, N. Theoretical investigation on the restoring step of the carbonic anhydrase catalytic cycle for natural and promiscuous substrates. Arch. Biochem. Biophys. 2014, 582, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Karplus, M. Is a “Proton Wire” Concerted or Stepwise? A Model Study of Proton Transfer in Carbonic Anhydrase. J. Phys. Chem. B 2003, 4, 1071–1078. [Google Scholar] [CrossRef]
- Silverman, D.; Lindskog., S. The catalytic mechanism of carbonic anhydrase: Implications of a rate-limiting protolysis of water. Acc. Chem. Res. 1988, 21, 30–36. [Google Scholar] [CrossRef]
- Dapprich, S.; Komaromi, I.; Byun, K.S.; Morokuma, K.; Frisch, M.J. A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J. Mol. Struct.-Theochem. 1999, 461, 1–21. [Google Scholar] [CrossRef]
- Morokuma, K.; Froese, R.D.; Dapprich, S.; Komaromi, I.; Khoroshun, D.; Byun, S.; Musaev, D.G.; Emerson, C.L. The ONIOM (our own integrated N-layered molecular orbital and molecular mechanics) method, and its applications to calculations of large molecular systems. Abstr. Pap. Am. Chem. Soc. 1998, 215, U218. [Google Scholar]
- Morokuma, K.; Wang, Q.; Vreven, T. Performance Evaluation of the Three-Layer ONIOM Method: Case Study for a Zwitterionic Peptide. J. Chem. Theory Comput. 2006, 2, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Vreven, T.; Byun, K.S.; Komáromi, I.; Dapprich, S.; Montgomery, J.A., Jr.; Morokuma, K.; Frisch, M.J.J. Combining Quantum Mechanics Methods with Molecular Mechanics Methods in ONIOM. Chem. Theory Comput. 2006, 2, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Rappé, A.K.; Goddard, W.A., III. Charge equilibration for molecular dynamics simulations. J. Phys. Chem. 1991, 95, 3358–3363. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter Mater. Phys. 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Piazzetta, P.; Marino, T.; Russo, N.; Salahub, D.R. Direct Hydrogenation of Carbon Dioxide by an Artificial Reductase Obtained by Substituting Rhodium for Zinc in the Carbonic Anhydrase Catalytic Center. A Mechanistic Study. ACS Catal. 2015, 5, 5397–5409. [Google Scholar] [CrossRef]
- Amata, O.; Marino, T.; Russo, N.; Toscano, M. Catalytic activity of a ζ-class zinc and cadmium containing carbonic anhydrase. Compared work mechanisms. Phys. Chem. Chem. Phys. 2011, 13, 3468–3477. [Google Scholar] [CrossRef] [PubMed]
- Andrae, D.; Haussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta. 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed]
- Piazzetta, P.; Marino, T.; Russo, N.; Salahub, D.R. Explicit waters play a key role in the mechanism of rhodium-substituted human carbonic anhydrase. Chem. Cat. Chem. 2017, 9, 1047–1053. [Google Scholar] [CrossRef]
- Cornell, W.D.; Cieplak, P.; Bayly, C.I.; Gould, I.R.; Merz, K.M., Jr.; Ferguson, D.M.; Spellmeyer, D.C.; Fox, T.; Caldwell, J.W.; Kollman, P.A.; et al. A second generation force-field for the simulation of proteins, nucleic-acids, and organic-molecules. J. Am. Chem. Soc. 1995, 117, 5179–5197. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Marenich, A.V.; Jerome, S.V.; Cramer, C.J.; Truhlar, D.G. Charge Model 5: An Extension of Hirshfeld Population Analysis for the Accurate Description of Molecular Interactions in Gaseous and Condensed Phases. J. Chem. Theory Comput. 2012, 8, 527–541. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |









© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piazzetta, P.; Marino, T.; Russo, N. Mechanistic Explanation of the Weak Carbonic Anhydrase’s Esterase Activity. Molecules 2017, 22, 1009. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22061009
Piazzetta P, Marino T, Russo N. Mechanistic Explanation of the Weak Carbonic Anhydrase’s Esterase Activity. Molecules. 2017; 22(6):1009. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22061009
Chicago/Turabian StylePiazzetta, Paolo, Tiziana Marino, and Nino Russo. 2017. "Mechanistic Explanation of the Weak Carbonic Anhydrase’s Esterase Activity" Molecules 22, no. 6: 1009. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22061009