BET & ELF Quantum Topological Analysis of Neutral 2-Aza-Cope Rearrangement of γ-Alkenyl Nitrones
Abstract
:1. Introduction
2. Computational Methods
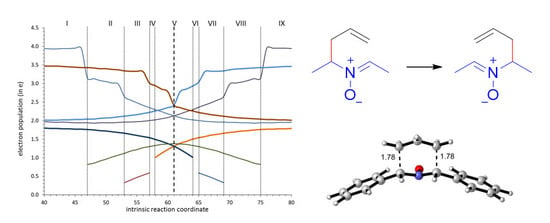
3. Results
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 1994, 371, 683. [Google Scholar] [CrossRef]
- Domingo, L.R. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R. A new C-C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Poater, J.; Duran, M.; Sola, M.; Silvi, B. Theoretical Evaluation of Electron Delocalization in Aromatic Molecules by Means of Atoms in Molecules (AIM) and Electron Localization Function (ELF) Topological Approaches. Chem. Rev. 2005, 105, 3911–3947. [Google Scholar] [CrossRef] [PubMed]
- Andres, J.; Berski, S.; Domingo, L.R.; Polo, V.; Silvi, B. Describing the Molecular Mechanism of Organic Reactions by Using Topological Analysis of Electronic Localization Function. Curr. Org. Chem. 2011, 15, 3566–3575. [Google Scholar] [CrossRef]
- Andres, J.; Berski, S.; Silvi, B. Curly arrows meet electron density transfers in chemical reaction mechanisms: from electron localization function (ELF) analysis to valence-shell electron-pair repulsion (VSEPR) inspired interpretation. Chem. Commun. 2016, 52, 8183–8195. [Google Scholar] [CrossRef] [PubMed]
- Merino, P.; Chiacchio, M.A.; Legnani, L.; Delso, I.; Tejero, T. Introducing Topology to Assess Synchronicity of Organic Reactions. Dual Reactivity of Oximes with Alkenes as a Case Study. Org. Chem. Front. 2017. [Google Scholar] [CrossRef]
- Roca-López, D.; Polo, V.; Tejero, T.; Merino, P. Mechanism Switch in Mannich-type Reactions. ELF and NCI Topological Analyses of the Reaction between Nitrones and Lithium Enolates. Eur. J. Org. Chem. 2015, 4143–4152. [Google Scholar] [CrossRef]
- Roca-López, D.; Polo, V.; Tejero, T.; Merino, P. Understanding Bond Formation in Polar One-Step Reactions. Topological analyses of the Reaction between Nitrones and Lithium Ynolates. J. Org. Chem. 2015, 80, 4076–4083. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Chamorro, E.; Perez, P. Understanding the mechanism of non-polar Diels–Alder reactions. A comparative ELF analysis of concerted and stepwise diradical mechanisms. Org. Biomol. Chem. 2010, 8, 5495–5504. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Picher, M.T.; Arroyo, P.; Saez, J.A. 1,3-Dipolar Cycloadditions of Electrophilically Activated Benzonitrile N-Oxides. Polar Cycloaddition versus Oxime Formation. J. Org. Chem. 2006, 71, 9319–9330. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Rios-Gutierrez, M.; Perez, P. A molecular electron density theory study of the [3 + 2] cycloaddition reaction of nitrones with strained allenes. RSC Adv. 2017, 7, 26879–26887. [Google Scholar] [CrossRef]
- Thom, R. Stabilité Structurelle et Morphogénèse; Intereditions: Paris, France, 1972. [Google Scholar]
- Krokidis, X.; Noury, S.; Silvi, B. Characterization of Elementary Chemical Processes by Catastrophe Theory. J. Phys. Chem. A 1997, 101, 7277–7282. [Google Scholar] [CrossRef]
- Polo, V.; Andres, J.; Berski, S.; Domingo, L.R.; Silvi, B. Understanding Reaction Mechanisms in Organic Chemistry from Catastrophe Theory Applied to the Electron Localization Function Topology. J. Phys. Chem. A 2008, 112, 7128–7136. [Google Scholar] [CrossRef] [PubMed]
- Andrés, J.; Gracia, L.; González-Navarrete, P.; Safont, V.S. Chemical structure and reactivity by means of quantum chemical topology analysis. Comput. Theor. Chem. 2015, 1053, 17–30. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Darù, A.; Tejero, T.; Domingo, L.R.; Merino, P. A Molecular Electron Density Theory Study of the [3+2] Cycloaddition Reaction of Nitrones with Ketenes. Org. Biomol. Chem. 2017, 15, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Rios-Gutierrez, M. A Molecular Electron Density Theory Study of the Reactivity of Azomethine Imine in [3+2] Cycloaddition Reactions. Molecules 2017, 22, 750–770. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Rios-Gutierrez, M.; Perez, P.; Chamorro, E. Understanding the [2n+2n] reaction mechanism between a carbenoid intermediate and CO2. Mol. Phys. 2016, 114, 1374–1391. [Google Scholar] [CrossRef]
- Rios-Gutierrez, M.; Perez, P.; Domingo, L.R. A bonding evolution theory study of the mechanism of [3+2] cycloaddition reactions of nitrones with electron-deficient ethylenes. RSC Adv. 2015, 5, 58464–58477. [Google Scholar] [CrossRef]
- Berski, S.; Ciunik, L.Z. The mechanism of the formation of the hemiaminal and Schiff base from the benzaldehyde and triazole studied by means of the topological analysis of electron localisation function and catastrophe theory. Mol. Phys. 2015, 113, 765–781. [Google Scholar] [CrossRef]
- Polo, V.; Andres, J.; Castillo, R.; Berski, S.; Silvi, B. Understanding the Molecular Mechanism of the 1,3-Dipolar Cycloaddition between Fulminic Acid and Acetylene in Terms of the Electron Localization Function and Catastrophe Theory. Chem. Eur. J. 2004, 10, 5165–5172. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Rios-Gutierrez, M.; Perez, P. An MEDT study of the carbenoid-type 3+2 cycloaddition reactions of nitrile ylides with electron-deficient chiral oxazolidinones. Org. Biomol. Chem. 2016, 14, 10427–10436. [Google Scholar] [CrossRef] [PubMed]
- Muller, P. Glossary of terms used in physical organic chemistry (IUPAC Recommendations 1994). Pure Appl. Chem. 2009, 66, 1077–1184, (on page 1104). [Google Scholar] [CrossRef]
- Rhoads, S.J.; Raulins, N.R. The Claisen and Cope Rearrangements. Org. React. (N. Y.) 1975, 22, 1. [Google Scholar]
- Hill, R.K. Cope, Oxy-Cope and Anionic Oxy-Cope Rearrangements. In Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon Press: Oxford, UK, 1991; Volume 5, p. 785. [Google Scholar]
- Polo, V.; Andres, J. A joint study based on the electron localization function and catastrophe theory of the chameleonic and centauric models for the Cope rearrangement of 1,5-hexadiene and its cyano derivatives. J. Comput. Chem. 2005, 26, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Blechert, S. The Hetero-Cope Rearrangement in Organic Synthesis. Synthesis 1989, 71. [Google Scholar] [CrossRef]
- Rehbein, J.; Hiersemann, M. Claisen Rearrangement of Aliphatic Allyl Vinyl Ethers from 1912 to 2012: 100 Years of Electrophilic Catalysis. Synthesis 2013, 45, 1121–1159. [Google Scholar] [CrossRef]
- Wipf, P. Claisen Rearrangements. In Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon Press: Oxford, UK, 1991; Volume 5, p. 827. [Google Scholar]
- Chamorro, E.; Santos, J.C.; Gomez, B.; Contreras, R.; Fuentealba, P. The Bonding Nature of Some Simple Sigmatropic Transition States from the Topological Analysis of the Electron Localization Function. J. Phys. Chem. A 2002, 106, 11533–11539. [Google Scholar] [CrossRef]
- Chamorro, E.; Santos, J.C.; Gomez, B.; Contreras, R.; Fuentealba, P. Topological analysis of the electron localization function applied to the study of the [1,3] sigmatropic shift of fluorine in 3-fluoropropene. J. Chem. Phys. 2001, 114, 23–34. [Google Scholar] [CrossRef]
- Berski, S.; Durlak, P. The mechanism of Claisen rearrangement of allyl phenyl ether from the perspective of topological analysis of the ELF. New J. Chem. 2016, 40, 8717–8726. [Google Scholar] [CrossRef]
- Hoffmann, R.W.; Endesfelder, A. Stereoselective Intramolecular Nitrone Cycloaddition in the Synthesis of Lasubine-Ii. Liebigs Annalen Der Chemie 1986, 1823–1836. [Google Scholar] [CrossRef]
- Merino, P.; Tejero, T.; Mannucci, V. Experimental and theoretical evidences of 2-aza-Cope rearrangement of nitrones. Tetrahedron Lett. 2007, 48, 3385–3388. [Google Scholar] [CrossRef]
- Delso, I.; Melicchio, A.; Isasi, A.; Tejero, T.; Merino, P. Evasive Neutral 2-Aza-Cope Rearrangements. Kinetic and Computational Studies with Cyclic Nitrones. Eur. J. Org. Chem. 2013, 2013, 5721–5730. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09. Revision D1; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6–31G* basis set for third-row atoms. J. Comput. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Fukui, K. Formulation of the reaction coordinate. J. Phys. Chem. 1970, 74, 4161–4163. [Google Scholar] [CrossRef]
- Fukui, K. The path of chemical reactions—The IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- González, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- González, C.; Schlegel, H.B. Improved algorithms for reaction path following: Higher-order implicit algorithms. J. Chem. Phys. 1991, 95, 5853–5860. [Google Scholar] [CrossRef]
- Savin, A.; Becke, A.D.; Flad, J.; Nesper, R.; Preuss, H.; Vonschnering, H.G. Angew. Chem. Int. Ed. 1991, 30, 409–412. [CrossRef]
- Savin, A.; Nesper, R.; Wengert, S.; Fassler, T.F. ELF: The Electron Localization Function. Angew. Chem. Int. Ed. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Noury, S.; Krokidis, X.; Fuster, F.; Silvi, B. Computational tools for the electron localization function topological analysis. Comput. Chem. 1999, 23, 597–604. [Google Scholar] [CrossRef]
- Legault, C.Y. CYLview, 1.0b. Université de Sherbrooke. 2009. Available online: http://www.cylview.org (accessed on 16 August 2017).
- The division of fragments is a conceptual idea since the molecule is not broken. Actually, such a division represents an arrangement of the molecule in two sub-groups just for discussion. GEDT values have been evaluated in the TS where the two fragments can be clearly identified as separate sub-units of a single molecule.
Sample Availability: Not Available. |










| Nitrone b | R1 | R2 | R3 | R4 | Barrier c | GEDT d | C1–C6 e | C3–C4 e |
|---|---|---|---|---|---|---|---|---|
| 1 | Ph | Ph | H | H | 32.7 | 0.09 | 1.78 | 1.78 |
| 2 | Ph | 4-NO2C6H4 | H | H | 32.9 | 0.11 | 1.79 | 1.78 |
| 3 | Ph | 4-MeOC6H4 | H | H | 33.5 | 0.08 | 1.78 | 1.79 |
| 4 | Ph | Me | H | H | 28.6 | 0.06 | 1.71 | 1.84 |
| 5 | Ph | CF3 | H | H | 26.8 | 0.11 | 1.69 | 1.88 |
| 6 | Ph | CH2OMe | H | H | 27.5 | 0.08 | 1.70 | 1.85 |
| (E)-7 | Ph | Ph | tBu | H | 43.6 | 0.10 | 1.88 | 1.88 |
| (Z)-7 | Ph | Ph | tBu | H | 46.5 | 0.10 | 2.17 | 2.13 |
| (E)-8 | Ph | Ph | OMe | H | 36.9 | 0.11 | 1.88 | 1.94 |
| (Z)-8 | Ph | Ph | OMe | H | 42.4 | 0.05 | 1.84 | 1.84 |
| (E)-9 | Ph | Ph | NO2 | H | 36.0 | −0.02 | 1.84 | 1.88 |
| (Z)-9 | Ph | Ph | NO2 | H | 43.0 | −0.08 | 1.87 | 1.88 |
| 10 | Ph | Ph | H | OMe | 34.1 | 0.17 | 1.86 | 1.68 |
| (E)-11 | NO2 | Me | OMe | H | 31.9 | 0.14 | 1.66 | 1.79 |
| (Z)-11 | NO2 | Me | OMe | H | 39.1 | 0.08 | 1.66 | 1.79 |
| 12 | H | COOMe | 34.4 | 0.07 | 1.79 | 1.70 | ||
| 13 | CH2OMe | COOMe | 30.9 | 0.08 | 1.75 | 1.75 |
| P1f a | P47 | P53 | P57 | P58 | P64 | P65 | P69 | P75 | P121 a | TS b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | ||||||||||||
| d(C1,C6) | 3.654 | 2.405 | 2.128 | 1.944 | 1.899 | 1.702 | 1.685 | 1.633 | 1.590 | 1.552 | 1.781 | |||||||||
| d(C3,C4) | 1.552 | 1.590 | 1.634 | 1.685 | 1.702 | 1.890 | 1.944 | 2.129 | 2.405 | 3.654 | 1.782 | |||||||||
| d(C1,N2) | 1.316 | 1.323 | 1.346 | 1.376 | 1.385 | 1.434 | 1.44 | 1.461 | 1.483 | 1.506 | 1.411 | |||||||||
| d(N2,C3) | 1.506 | 1.483 | 1.461 | 1.441 | 1.434 | 1.385 | 1.376 | 1.347 | 1.323 | 1.316 | 1.412 | |||||||||
| d(C5,C6) | 1.333 | 1.343 | 1.362 | 1.389 | 1.397 | 1.448 | 1.454 | 1.476 | 1.494 | 1.506 | 1.424 | |||||||||
| d(C4,C5) | 1.506 | 1.494 | 1.475 | 1.454 | 1.447 | 1.397 | 1.389 | 1.362 | 1.342 | 1.333 | 1.424 | |||||||||
| V(C1,C6) | 1.01 | 1.50 | 1.54 | 1.66 | 1.76 | 1.85 | 1.31 | |||||||||||||
| V(C3,C4) | 1.85 | 1.76 | 1.66 | 1.54 | 1.49 | 1.01 | 1.31 | |||||||||||||
| V1(C4,C5) | 1.99 | 2.04 | 2.12 | 2.22 | 2.26 | 2.93 | 3.04 | 3.34 | 1.67 | 1.75 | 2.42 | |||||||||
| V2(C4,C5) | 1.76 | |||||||||||||||||||
| V1(C5,C6) | 1.75 | 1.67 | 3.34 | 3.02 | 2.94 | 2.26 | 2.22 | 2.12 | 2.04 | 1.99 | 2.42 | |||||||||
| V2(C5,C6) | 1.76 | 1.76 | ||||||||||||||||||
| V(C1,N2) | 3.96 | 3.14 | 2.64 | 2.35 | 2.29 | 2.02 | 2.00 | 1.96 | 1.94 | 1.98 | 2.13 | |||||||||
| V(C3,N2) | 1.98 | 1.94 | 1.96 | 2.00 | 2.02 | 2.29 | 2.36 | 2.64 | 3.14 | 3.96 | 2.13 | |||||||||
| V(C1) | 0.33 | 0.59 | ||||||||||||||||||
| V(N2) | 0.82 | 1.12 | 1.30 | 1.33 | 1.33 | 1.30 | 1.12 | 0.82 | 1.38 | |||||||||||
| V(C3) | 0.33 | 0.59 | ||||||||||||||||||
| V(C4) | 0.20 | |||||||||||||||||||
| V(C5) | 0.29 | |||||||||||||||||||
| V(C6) | 0.29 | |||||||||||||||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merino, P.; Chiacchio, M.A.; Legnani, L.; Tejero, T. BET & ELF Quantum Topological Analysis of Neutral 2-Aza-Cope Rearrangement of γ-Alkenyl Nitrones. Molecules 2017, 22, 1371. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22081371
Merino P, Chiacchio MA, Legnani L, Tejero T. BET & ELF Quantum Topological Analysis of Neutral 2-Aza-Cope Rearrangement of γ-Alkenyl Nitrones. Molecules. 2017; 22(8):1371. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22081371
Chicago/Turabian StyleMerino, Pedro, Maria A. Chiacchio, Laura Legnani, and Tomás Tejero. 2017. "BET & ELF Quantum Topological Analysis of Neutral 2-Aza-Cope Rearrangement of γ-Alkenyl Nitrones" Molecules 22, no. 8: 1371. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22081371