4.3. Structure Identification of Compounds
Melting points were determined in open capillary tubes. The
1H-NMR and
13C-NMR spectra were measured on an AV-400 spectrometer (Bruker, Karlsruhe, Germany) in CDCl
3 solutions using tetramethylsilane as the internal standard. Mass spectra were obtained on a Quattro Micromass instrument (Waters, Milford, MA, USA). As shown in our previous work, the hydroxyl groups of 25-OCH
3–PPD, 25-OH-PPD or PD were replaced. In the
13C-NMR spectrum, the signals changed from δC 78.0–79.0 (C-3), 70.5–71.5 (C-12) to δC 81.0–83.0 (C-3), 75.0–77.0 (C-12), respectively. In the
1H-NMR spectrum, signals changed from δH 3.2 (H-3), 3.6 (H-12) to δH 4.7–4.8 (H-3), 5.1–5.2 (H-12), respectively. Combining the spectrum of salicylic acid and acetylsalicylic acid, we could accurately determine the structures of the derivatives on the basis of 25-OH-PPD, 25-OCH
3-PPD or PD. The spectrums (see
Table 1) of all compounds are in
Supplementary Materials.
20(R)-Dammarane-20-hydroxy-3β,12β,25-tri-yl-2′-hydroxybenzoate (1a, C51H66O10). White solid, melting point: 192–194 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.94 (s, 3H), 0.95 (s, 3H), 1.02 (s, 3H), 1.09 (s, 3H), 1.59 (s, 6H), 1.62 (s, 6H), 4.77 (td, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, H-3), 5.20 (td, J1,3 = 12.0 Hz, J1,2 = 8.0 Hz, 1H, H-12), 6.82–6.89 (m, 3H, Ar-H), 6.97 (t, J1,2 = 8 Hz, J1,3 = 20.0 Hz, 3H, Ar-H), 7.44 (td, J1,3 = 8.0 Hz, J1,2 = 8.0 Hz, 3H, Ar-H), 7.81 (dd, J1,3 = 28.0 Hz, J1,2 = 8.0 Hz, 3H, Ar-H), 10.79 (s, 1H, AR-OH), 10.92 (s, 1H, Ar-OH), 11.04 (s, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 39.85 (C-1), 26.44 (C-2), 82.04 (C-3), 38.40 (C-4), 56.05 (C-5), 18.16 (C-6), 34.55 (C-7), 38.61 (C-8), 51.75 (C-9), 37.25 (C-10), 33.20 (C-11), 77.37 (C-12), 49.94 (C-13), 53.46 (C-14), 31.11 (C-15), 26.36 (C-16), 52.91(C-17), 16.87 (C-18), 15.82 (C-19), 73.93 (C-20), 23.69 (C-21), 41.87 (C-22), 18.30 (C-23), 44.97 (C-24), 85.34 (C-25), 28.48 (C-26), 28.30 (C-27), 26.91 (C-28), 16.28 (C-29), 17.28 (C-30). Structure of salicylic acid: 112.71, 113.14, 113.93 (C-1), 117.66, 117.74, 118.07 (C-3), 119.08, 119.18, 119.32 (C-5), 129.36, 129.85, 130.23 (C-6), 135.37, 135.63, 136.02 (C-4), 161.88, 161.91, 162.13 (C-2), 169.40, 169.94, 170.04 (–C=O). MS: m/z 838.47 [M + H]+.
20(R)-25-Methoxydammarane-20-hydroxy-3β,12β-di-yl-2′-hydroxybenzoate (2a, C45H64O8). White solid, melting point: 200–202 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.94 (s, 6H), 1.02 (s, 3H), 1.03 (s, 3H), 1.08 (s, 3H), 1.09 (s, 3H), 1.14 (s, 3H), 1.16 (s, 3H), 4.75–4.79 (dd, J1,3 = 12.0 Hz, 11H, H-3), 5.18–5.25 (td, J1,3 = 12.0 Hz, J1,2 = 8.0 Hz, 1H, H-12), 6.84–6.88 (td, J1,3 = 12.0 Hz, J1,2 = 4.0 Hz, 2H, Ar-H), 6.94–6.98 (t, J1,3 = 16.0 Hz, J1,2 = 8.0 Hz, 2H, Ar-H), 7.41–7.46 (m, 2H, Ar-H), 7.73–7.82 (m, 2H, Ar-H), 10.82 (s, 1H, Ar-OH), 10.92 (s, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 38.57 (C-1), 28.25 (C-2), 82.01 (C-3), 39.82 (C-4), 56.00 (C-5), 18.24 (C-6), 34.51 (C-7), 38.30 (C-8), 49.90 (C-9), 37.22 (C-10), 31.08 (C-11), 77.28 (C-12), 49.26 (C-13), 53.27 (C-14), 30.75 (C-15), 26.44 (C-16), 52.35 (C-17), 16.24 (C-18), 16.85 (C-19), 73.99 (C-20), 23.66 (C-21), 44.96 (C-22), 18.01 (C-23), 41.13 (C-24), 74.73 (C-25), 25.21 (C-26), 24.91 (C-27), 28.45 (C-28), 15.78 (C-29), 17.26 (C-30). Structure of salicylic acid: 112.78, 113.10 (C-1), 117.70, 117.90 (C-3), 119.14, 119.25 (C-5), 129.42, 129.81 (C-6), 135.59, 135.87 (C-4), 161.85, 162.11 (C-2), 169.38, 169.90 (–C=O); MS: m/z 731.91 [M − H]+.
20(R)-25-Methoxydammarane-3β,20-diol-12β-yl-2′-hydroxybenzoate (3a, C38H60O6). White solid, melting point: 235–237 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.75 (s, 3H), 0.83 (s, 3H), 0.95 (s, 3H), 0.99 (s, 3H), 1.04 (s, 3H), 1.07 (s, 3H), 1.12 (s, 3H), 1.13 (s, 3H), 3.19 (dd, J1,2 = 4.0 Hz, J1,3 = 8.0 Hz, 1H, H-3), 5.17 (td, J1,3 = 12.0 Hz, J1,2 = 4.0 Hz, 1H, H-12), 6.84 (t, J1,3 = 12.0 Hz, J1,2 = 4.0 Hz, 1H, Ar-H), 6.94 (d, J1,2 = 8.0 Hz, 1H, Ar-H), 7.41 (td, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, Ar-H), 7.73 (dd, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, Ar-H), 10.81 (d, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 38.96 (C-1), 27.25 (C-2), 78.65 (C-3), 39.70 (C-4), 55.82 (C-5), 18.27 (C-6), 34.54 (C-7), 38.85 (C-8), 49.92 (C-9), 37.18 (C-10), 31.17 (C-11), 77.25 (C-12), 49.18 (C-13), 52.80 (C-14), 31.00 (C-15), 26.33 (C-16), 50.00 (C-17), 15.69 (C-18), 16.12 (C-19), 73.95 (C-20), 23.03 (C-21), 44.88 (C-22), 17.93 (C-23), 41.00 (C-24), 74.70 (C-25), 25.15 (C-26), 24.87 (C-27), 28.07 (C-28), 15.44 (C-29), 17.19 (C-30), 49.09 (–OCH3). Structure of salicylic acid: 112.72 (C-1), 117.90 (C-3), 119.22 (C-5), 129.39 (C-6), 135.82 (C-4), 161.98 (C-2), 169.39 (–C=O).
20(R)-25-Methoxydammarane-12β,20-diol-3β-yl-2′-hydroxybenzoate (4a, C38H60O6). White solid, melting point: 246–248 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.91 (s, 3H), 0.94 (s, 3H), 0.96 (s, 3H), 1.01 (s, 3H), 1.02 (s, 3H), 1.16 (s, 6H), 1.21 (s, 3H), 3.59–3.65 (td, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, H-12), 4.74–4.78 (dd, J1,3 = 12.0 Hz, J1,2 = 8.0 Hz, 1H, H-3), 6.85–6.90 (t, J1,3 = 16.0 Hz, J1,2 = 6.0 Hz, 1H, Ar-H), 6.97–6.99 (d, J1,2 = 8.0 Hz, 1H, Ar-H), 7.43–7.47 (td, J1,3 = 8.0 Hz, J1,2 = 3.0 Hz, 1H, Ar-H), 7.82–7.84 (dd, J1,3 = 8.0 Hz, J1,2 = 3.0 Hz, 1H, Ar-H), 10.93 (s, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 38.72 (C-1), 27.24 (C-2), 82.32 (C-3), 39.92 (C-4), 56.12 (C-5), 18.31 (C-6), 34.82 (C-7), 38.38 (C-8), 50.09 (C-9), 37.20 (C-10), 31.30 (C-11), 70.95 (C-12), 49.29 (C-13), 53.61 (C-14), 31.13 (C-15), 26.63 (C-16), 51.72 (C-17), 16.33 (C-18), 16.87 (C-19), 74.63 (C-20), 23.82 (C-21), 42.82 (C-22), 17.88 (C-23), 40.68 (C-24), 74.87 (C-25), 25.25 (C-26), 25.12 (C-27), 28.29 (C-28), 15.80 (C-29), 16.97 (C-30), 47.95 (OCH3). Structure of salicylic acid: 113.17 (C-1), 117.71 (C-3), 119.20 (C-5), 129.89 (C-6), 135.61 (C-4), 161.85 (C-2), 170.03 (–C=O). MS: m/z 612.88 [M + H]+.
20(R)-Dammarane-3β,20,25-triol-12β-yl-2′-hydroxybenzoate (5a, C37H58O6). White solid, melting point: 258–260 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.77 (s, 3H), 0.85 (s, 3H), 0.97 (s, 3H), 1.02 (s, 6H), 1.06 (s, 3H), 1.15, (s, 6H), 1.18 (s, 3H), 3.18–3.22 (dd, J1,3 = 12.0 Hz, J1,2 = 4.0 Hz, 1H, H-3), 5.16–5.22 (td, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, H-12), 6.87–6.91 (t, J1,3 = 16.0 Hz, J1,2 = 8.0 Hz, 1H, Ar-H), 6.94–6.96 (d, J1,2 = 8.0 Hz, 1H, Ar-H), 7.41–7.45 (td, J1,3 = 8.0 Hz, J1,2 = 2.8 Hz, 1H, Ar-H), 7.76–7.79 (dd, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, Ar-H), 10.83 (s, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 39.68 (C-1), 28.00 (C-2), 78.68 (C-3), 38.90 (C-4), 55.78 (C-5), 17.76 (C-6), 37.14 (C-7), 42.64 (C-8), 50.00 (C-9), 38.80 (C-10), 33.58 (C-11), 77.48 (C-12), 49.45 (C-13), 53.47 (C-14), 31.19 (C-15), 27.09 (C-16), 52.84 (C-17), 15.62 (C-18), 16.10 (C-19), 74.01 (C-20), 23.00 (C-21), 44.26 (C-22), 18.22 (C-23), 45.43 (C-24), 71.26 (C-25), 29.28 (C-26), 29.02 (C-27), 28.38 (C-28), 15.39 (C-29), 17.59 (C-30). Structure of salicylic acid: 112.63 (C-1), 117.83 (C-3), 119.47 (C-5), 129.37 (C-6), 135.87 (C-4), 161.90 (C-2), 169.42 (–C=O). MS: m/z 598.85 [M + H]+.
20(R)-Dammarane-12β, 20-diol-3β, 25-di-yl-2′-hydroxybenzoate (6a, C44H62O8). White solid, melting point: 219–221 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.84 (s, 3H), 0.93 (s, 3H), 0.95 (s, 3H), 1.00 (s, 3H), 1.02 (s, 3H), 1.14 (s, 3H), 1.60 (s, 3H), 1.62 (s, 3H), 3.59–3.66 (td, J1,3 = 12.0 Hz, 1H, H-12), 4.73–4.77 (dd, J1,3 = 12.0 Hz, J1,2 = 8.0 Hz, 1H, H-3), 6.82–6.89 (m, 2H, Ar-H), 6.94–6.99 (m, 2H, Ar-H), 7.39–7.47 (m, 2H, Ar-H), 7.76–7.84 (m, 2H, Ar-H), 10.92 (s, 1H, Ar-OH), 11.02 (s, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 39.91 (C-1), 26.51 (C-2), 82.29 (C-3), 38.40 (C-4), 56.12 (C-5), 18.31 (C-6), 34.80 (C-7), 41.61 (C-8), 51.02 (C-9), 37.20 (C-10), 31.57 (C-11), 71.19 (C-12), 49.96 (C-13), 53.56 (C-14), 31.09 (C-15), 26.31 (C-16), 51.80 (C-17), 16.34 (C-18), 16.87 (C-19), 74.31 (C-20), 23.81 (C-21), 43.00 (C-22), 22.33 (C-23), 48.48 (C-24), 85.28 (C-25), 28.77 (C-26), 28.30 (C-27), 27.73 (C-28), 15.82 (C-29), 17.68 (C-30). Structure of salicylic acid: 113.18, 113.93 (C-1), 117.68, 117.74 (C-3), 119.05, 119.22 (C-5), 129.90, 130.20 (C-6), 135.35, 135.64 (C-4), 161.87, 161.93 (C-2), 169.93, 170.05 (–C=O). MS: m/z 718.96 [M + H]+.
20(R)-Dammarane-20, 25-diol-3β, 12β-di-yl-2′-hydroxybenzoate (7a, C44H62O8). White solid, melting point: 197–199 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.95 (s, 6H), 1.02 (s, 3H), 1.05 (s, 3H), 1.09 (s, 3H), 1.17 (s, 6H), 1.19 (s, 3H), 4.76–4.80 (dd, J1,3 = 12.0 Hz, J1,2 = 4.0 Hz, 1H, H-3), 5.19–5.25 (td, J1,3 = 8.0 Hz, J1,3 = 4.0 Hz, 1H, H-12), 6.85–6.92 (m, 2H, Ar-H), 6.96–6.99 (m, 2H, Ar-H), 7.42–7.46 (m, 2H, Ar-H), 7.77–7.83 (m, 2H, Ar-H), 10.92 (s, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 38.61 (C-1), 28.28 (C-2), 82.03 (C-3), 38.39 (C-4), 56.06 (C-5), 17.93 (C-6), 34.55 (C-7), 39.87 (C-8), 49.60 (C-9), 37.26 (C-10), 33.80 (C-11), 77.25 (C-12), 49.53 (C-13), 52.96 (C-14), 31.35 (C-15), 26.97 (C-16), 50.06 (C-17), 16.30 (C-18), 16.86 (C-19), 73.95 (C-20), 23.16 (C-21), 44.47 (C-22), 18.28 (C-23), 45.59 (C-24), 71.23 (C-25), 29.52 (C-26), 28.25 (C-27), 28.57 (C-28), 15.79 (C-29), 17.75 (C-30). Structure of salicylic acid: 112.75, 113.13 (C-1), 117.73, 118.00 (C-3), 119.17, 119.58 (C-5), 129.45, 129.83 (C-6), 135.62, 136.00 (C-4), 161.85, 162.09 (C-2), 169.52, 169.93 (–C=O).
20(R)-Dammarane-3β,12β,20-triol-25-yl-2′-hydroxybenzoate (8a, C37H58O6). White solid, melting point: 223–225 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.74 (s, 3H), 0.79 (s, 3H), 0.82 (s, 3H), 0.94 (s, 6H), 1.08 (s, 3H), 1.56 (s, 3H), 1.58 (s, 3H), 3.14–3.18 (dd, J1,3 = 12.0 Hz, J1,2 = 4.0 Hz, 1H, H-3), 3.52–3.58 (td, J1,3 = 12.0 Hz, J1,2 = 4.0 Hz, 1H, H-12), 6.78–6.82 (t, J1,3 = 16.0 Hz, J1,2 = 8.0 Hz, 1H, Ar-H), 6.91–6.93 (d, J = 8.0 Hz, 1H, Ar-H), 7.36–7.43 (m, 1H, Ar-H), 8.00–8.07 (dd, J1,3 = 20.0 Hz, J1,2 = 8.0 Hz, 1H, Ar-H), 11.03 (s, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 39.70 (C-1), 26.08 (C-2), 78.88 (C-3), 38.93 (C-4), 55.84 (C-5), 17.56 (C-6), 34.76 (C-7), 41.60 (C-8), 50.08 (C-9), 37.06 (C-10), 33.62 (C-11), 71.04 (C-12), 49.65 (C-13), 53.52 (C-14), 31.19 (C-15), 24.87 (C-16), 51.59 (C-17), 15.63 (C-18), 16.18 (C-19), 74.14 (C-20), 22.00 (C-21), 42.64 (C-22), 18.28 (C-23), 48.22 (C-24), 85.27 (C-25), 28.05 (C-26), 27.24 (C-27), 26.33 (C-28), 15.45 (C-29), 17.03 (C-30). Structure of salicylic acid: 113.84 (C-1), 117.52 (C-3), 118.07 (C-5), 130.12 (C-6), 135.22 (C-4), 161.74 (C-2), 169.83 (–C=O). MS: m/z 597.45 [M − H]+.
20(R)-Dammarane-12β,20,25-triol-3β-yl-2′-hydroxybenzoate (9a, C37H58O6). White solid, melting point: 295–297 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.91 (s, 3H), 0.94 (s, 3H), 0.96 (s, 3H), 1.01 (s, 3H), 1.02 (s, 3H), 1.14 (s, 3H), 1.24 (s, 6H), 3.63 (td, J1,3 = 10.2 Hz, J1,2 = 5.36 Hz, 1H, H-12), 4.75 (dd, J1,3 = 10.2 Hz, J1,2 = 5.88 Hz, 1H, H-3), 6.88 (t, J1,3 = 14.84 Hz, J1,2 = 7.4 Hz, 1H, Ar-H), 6.99 (d, J = 8.28 Hz, 1H, Ar-H), 7.44 (td, J1,3 = 8.52 Hz, J1,2 = 1.48 Hz, 1H, Ar-H), 7.83 (dd, J1,3 = 7.6 Hz, J1,2 = 1.52 Hz, 1H, Ar-H), 10.93 (s, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 38.74 (C-1), 28.30 (C-2), 82.32 (C-3), 38.39 (C-4), 56.13 (C-5), 17.85 (C-6), 34.82 (C-7), 39.92 (C-8), 50.11 (C-9), 37.21 (C-10), 31.45 (C-11), 71.29 (C-12), 48.65 (C-13), 53.57 (C-14), 31.16 (C-15), 36.52 (C-16), 51.78 (C-17), 16.35 (C-18), 16.88 (C-19), 74.57 (C-20), 22.04 (C-21), 43.12 (C-22), 18.32 (C-23), 44.28 (C-24), 71.00 (C-25), 29.84 (C-26), 29.59 (C-27), 29.55 (C-28), 15.81 (C-29), 17.30 (C-30). Structure of salicylic acid: 113.18 (C-1), 117.72 (C-3), 119.91 (C-5), 129.91 (C-6), 135.63 (C-4), 161.87 (C-2), 170.04 (–C=O).
20(R)-Pananxadiol-3β,12β-di-yl-2′-hydroxybenzoate (10a, C44H60O7). White solid, melting point: 232–234 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.65 (s, 3H), 0.91 (s, 3H), 0.92 (s, 3H), 0.94 (s, 3H), 1.00 (s, 3H), 1.01 (s, 3H), 1.05 (s, 3H), 1.16 (s, 3H), 4.75–4.85 (m, 2H, H-12, H-3), 6.85–6.89 (t, J1,3 = 15.36 Hz, J1,2 = 8.62 Hz, 2H, Ar-OH), 6.95–6.98 (d, J = 12.0 Hz, 2H, Ar-H), 7.64–7.69 (td, J1,2 = 4.0 Hz, J1,3 = 8.0Hz, 2H, Ar-H), 7.98–8.08 (m, 2H, Ar-H), 10.92 (s, 1H, Ar-OH), 11.19 (s, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 38.52 (C-1), 28.31 (C-2), 82.14 (C-3), 38.39 (C-4), 55.96 (C-5), 18.31 (C-6), 34.29 (C-7), 39.69 (C-8), 49.55 (C-9), 37.37 (C-10), 30.44 (C-11), 75.70 (C-12), 49.10 (C-13), 51.92 (C-14), 31.42 (C-15), 25.24 (C-16), 54.35 (C-17), 15.96 (C-18), 16.73 (C-19), 75.03 (C-20), 23.71 (C-21), 35.37 (C-22), 16.90 (C-23), 37.16 (C-24), 70.80 (C-25), 33.05 (C-26), 26.80 (C-27), 28.45 (C-28), 15.91 (C-29), 18.02 (C-30). Structure of salicylic acid: 113.16, 114.04 (C-1), 116.28, 117.72 (C-3), 118.94, 119.16 (C-5), 129.85, 130.30 (C-6), 135.59, 135.97 (C-4), 161.23, 161.87 (C-2), 169.78, 169.95 (C-7, –C=O); MS: m/z 700.94 [M + H]+.
20(R)-Pananxadiol-12β-hydroxy-3β-yl-2′-hydroxybenzoate (11a, C37H56O5). White solid, melting point: 222–224 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.90 (s, 3H), 0.93 (s, 3H), 0.95 (s, 3H), 1.00 (s, 3H), 1.01 (s, 3H), 1.19 (s, 3H), 1.22 (s, 3H), 1.27 (s, 3H), 3.52–3.58 (td, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, H-12), 4.73–4.77 (dd, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, H-3), 6.85–6.89 (t, J1,3 = 16.0 Hz, J1,2 = 8.0 Hz, 1H, Ar-H), 6.89–6.96 (d, J1,2 = 12.0 Hz, 1H, Ar-H), 7.42–7.46 (t, J1,3 = 16.0 Hz, J1,2 = 8.0 Hz, 1H, Ar-H), 7.82–7.84 (d, J1,2 = 8.0 Hz, 1H, Ar-H), 10.93 (s, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 38.70 (C-1), 27.29 (C-2), 82.45 (C-3), 39.39 (C-4), 56.17 (C-5), 18.36 (C-6), 34.94 (C-7), 39.98 (C-8), 50.00 (C-9), 37.24 (C-10), 30.72 (C-11), 70.02 (C-12), 49.31 (C-13), 51.30 (C-14), 31.28 (C-15), 23.89 (C-16), 54.87 (C-17), 16.32 (C-18), 16.42 (C-19), 76.67 (C-20), 19.57 (C-21), 35.89 (C-22), 16.85 (C-23), 36.59 (C-24), 73.27 (C-25), 33.17 (C-26), 25.31 (C-27), 28.30 (C-28), 15.80 (C-29), 17.20 (C-30). Structure of salicylic acid: 113.22 (C-1), 117.70 (C-3), 119.18 (C-5), 129.91 (C-6), 135.57 (C-4), 161.87 (C-2), 170.05 (–C=O); MS: m/z 603.42 [M + Na]+.
20(R)-Pananxadiol-3β-hydroxy-12β-yl-2′-hydroxybenzoate (12a, C37H58O6). White solid, melting point: 248–250 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.64 (s, 3H), 0.77 (s, 3H), 0.84 (s, 3H), 0.90 (s, 3H), 0.98 (s, 6H), 1.03 (s, 3H), 1.15 (s, 3H), 3.18–3.22 (dd, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, H-3), 6.85–6.89 (t, J1,3 = 16.0 Hz, J1,2 = 8.0 Hz, 1H, Ar-H), 6.95–6.98 (d, J1,2 = 12.0 Hz, 1H, Ar-H), 7.40–7.45 (td, J1,2 = 4.0 Hz, J1,3 = 8.0 Hz, 1H, Ar-H), 7.85–7.88 (dd, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, Ar-H), 11.20 (s, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 38.92 (C-1), 27.24 (C-2), 78.79 (C-3), 38.75 (C-4), 55.73 (C-5), 18.25 (C-6), 34.27 (C-7), 39.51 (C-8), 49.53 (C-9), 37.24 (C-10), 30.33 (C-11), 75.72 (C-12), 49.42 (C-13), 51.84 (C-14), 31.42 (C-15), 25.47 (C-16), 54.38 (C-17), 15.75 (C-18), 15.78 (C-19), 74.92 (C-20), 21.75 (C-21), 35.19 (C-22), 16.59 (C-23), 37.07 (C-24), 70.66 (C-25), 32.90 (C-26), 26.69 (C-27), 28.40 (C-28), 15.38 (C-29), 17.91 (C-30). Structure of salicylic acid: 113.93 (C-1), 117.53 (C-3), 118.78 (C-5), 130.20 (C-6), 135.18 (C-4), 161.70 (C-2), 169.68 (C-7, –C=O); MS: m/z 579.35 [M − H]+.
20(R)-Pananxadiol-3β-yl-2′-acetylbenzoate-12β-yl-2′-hydroxybenzoate (1b, C46H62O8). White solid, melting point: 198–200 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.65 (s, 3H), 0.91 (s, 6H), 0.93 (s, 3H), 0.97 (s, 3H), 0.99 (s, 3H), 1.05 (s, 3H), 1.15 (s, 3H), 4.69–4.73 (dd, J1,3 = 12.0 Hz, J1,2 = 4.0 Hz, 1H, H-3), 6.85–6.89 (t, J1,2 = 8.0 Hz, J1,3 = 16.0 Hz, 1H, Ar-H), 6.96–6.98 (d, J= 8.0 Hz, 1H, Ar-H), 7.08–7.10 (d, J = 8.0 Hz, 1H, Ar-H), 7.27–7.31 (t, J1,3 = 16.0 Hz, J1,2 = 8.0 Hz, 1H, Ar-H), 7.41–7.44 (t, J1,3 = 12.0 Hz, J1,2 = 6.2 Hz, 1H, Ar-H), 7.51–7.55 (t, J1,3 = 16.0 Hz, J1,3 = 8.0 Hz, 1H, Ar-H), 7.86–7.88 (d, J = 8.0 Hz, 1H, Ar-H), 7.96–7.98 (d, J = 8.0 Hz, 1H, Ar-H), 11.20 (s, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 38.56 (C-1), 28.22 (C-2), 81.42 (C-3), 38.32 (C-4), 56.00 (C-5), 18.25 (C-6), 33.04 (C-7), 39.65 (C-8), 49.55 (C-9), 37.36 (C-10), 30.42 (C-11), 75.72 (C-12), 49.12 (C-13), 51.89 (C-14), 31.28 (C-15), 25.57 (C-16), 54.53 (C-17), 15.97 (C-18), 16.71 (C-19), 75.02 (C-20), 21.69 (C-21), 34.30 (C-22), 16.91 (C-23), 37.14 (C-24), 70.78 (C-25), 32.18 (C-26), 26.78 (C-27), 28.52 (C-28), 15.80 (C-29), 17.98 (C-30). Structure of salicylic acid: 114.04 (C-1), 117.66 (C-3), 118.88 (C-5), 130.29 (C-6), 135.29 (C-4), 161.84 (C-2), 169.75 (C-7, –C=O); Structure of acetylsalicylic acid: 123.88 (C-8), 124.06 (C-7), 126.01 (C-6), 131.42 (C-5), 133.63 (C-4), 150.90 (C-3), 164.05 (C-2), 169.80 (C-1, C=O), 21.27 (C-9, –CH3). MS: m/z 742.98 [M + H]+.
20(R)-Pananxadiol-3β, 12β-di-yl-2′-acetylbenzoate (2b, C48H64O9). White solid, melting point: 238–240 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.74 (s, 3H), 0.91 (s, 3H), 0.92 (s, 3H), 0.96 (s, 3H), 0.98 (s, 3H), 1.01 (s, 3H), 1.03 (s, 3H), 1.13 (s, 3H), 4.69–4.73 (dd, J1,3 = 12.0 Hz, J1,2 = 4.0 Hz, 1H, H-3), 5.16–5.22 (td, J1,2 = 8.0 Hz, J1,3 = 12.0 Hz, 1H, H-12), 7.08–7.10 (dd, J1,3 = 8.0 Hz, 2H, Ar-H), 7.27–7.31 (td, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 2H, Ar-H), 7.51–7.55 (td, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 2H, Ar-H), 7.98–7.98 (dd, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, Ar-H), 8.06–8.09 (dd, J1,3 = 8.0 Hz, J1,3 = 4.0 Hz, 1H, Ar-H); 13C-NMR (CDCl3, 100 MHz, ppm): 38.52 (C-1), 28.21 (C-2), 81.46 (C-3), 38.32 (C-4), 56.00 (C-5), 18.25 (C-6), 34.35 (C-7), 39.67 (C-8), 49.63 (C-9), 37.31 (C-10), 30.68 (C-11), 75.41 (C-12), 45.30 (C-13), 52.06 (C-14), 30.79 (C-15), 25.80 (C-16), 53.63 (C-17), 16.02 (C-18), 16.63 (C-19), 75.18 (C-20), 19.29 (C-21), 34.60 (C-22), 16.90 (C-23), 37.16 (C-24), 70.70 (C-25), 33.00 (C-26), 27.22 (C-27), 28.51 (C-28), 15.89 (C-29), 18.20 (C-30); Structure of acetylsalicylic acid: 123.86, 123.96 (C-8), 124.07, 124.60 (C-7), 125.84, 125.98 (C-6), 131.01, 131.42 (C-5), 133.45, 133.59 (C-4), 150.87, 150.89 (C-3), 163.60, 164.03 (C-2), 169.64, 169.77 (C-1, C=O), 21.31, 21.25 (C-9, –CH3). MS: m/z 785.02 [M + H]+.
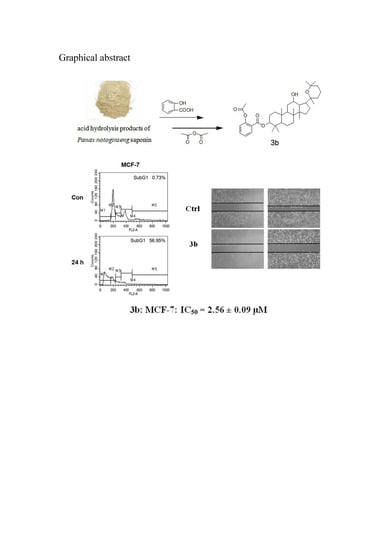
20(R)-Pananxadiol-12β-hydroxy-3β-yl-2′-acetylbenzoate (3b, C39H58O6). White solid, melting point: 243–245 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.89 (s, 3H), 0.91 (s, 3H), 0.94 (s, 3H), 0.97 (s, 3H), 0.99 (s, 3H), 1.19 (s, 3H), 1.22 (s, 3H), 1.27 (s, 3H), 3.52–3.58 (td, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, H-12), 4.67–4.71 (dd, J1,3 = 12.0 Hz, J1,2 = 4.0 Hz, 1H, H-3), 7.09–7.11 (d, J1,2 = 8.0 Hz, 1H, Ar-H), 7.28–7.32 (dd, J1,3 = 12.0 Hz, J1,2 = 8.0 Hz, 1H, Ar-H), 7.52–7.56(td, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, Ar-H), 7.97–7.99 (dd, J1,3 = 8.0 Hz, J1,2 = 3.6 Hz, 1H, Ar-H); 13C-NMR (CDCl3, 100 MHz, ppm): 38.77 (C-1), 27.30 (C-2), 81.76 (C-3), 38.34 (C-4), 56.23 (C-5), 18.36 (C-6), 34.98 (C-7), 39.99 (C-8), 50.03 (C-9), 37.24 (C-10), 30.71 (C-11), 70.04 (C-12), 49.32 (C-13), 51.35 (C-14), 31.28 (C-15), 25.32 (C-16), 54.88 (C-17), 16.37 (C-18), 16.42 (C-19), 76.67 (C-20), 19.57 (C-21), 35.90 (C-22), 16.89 (C-23), 36.60 (C-24), 73.25 (C-25), 33.17 (C-26), 27.21 (C-27), 28.24 (C-28), 15.80 (C-29), 17.19 (C-30). Structure of acetylsalicylic acid: 123.89 (C-8), 124.19 (C-7), 126.05 (C-6), 131.49 (C-5), 133.61 (C-4), 150.88 (C-3), 164.22 (C-2), 169.80 (C-1, C=O), 21.29 (C-9, CH3). MS: m/z 622.87 [M + H]+.
20(R)-Pananxadiol-3β-o-acetyl-12β-yl-2′-hydroxybenzoate (4b, C39H58O6). White solid, melting point: 238–240 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.85 (s, 3H), 0.86 (s, 3H), 0.87 (s, 3H), 0.90 (s, 3H), 0.96 (s, 3H), 0.98 (s, 3H), 1.03 (s, 3H), 1.15 (s, 3H), 4.47–4.51 (dd, J1,3 = 12.0 Hz, J1,2 = 4.0 Hz, 1H, H-3), 6.84–6.88 (t, J1,3 = 16.0 Hz, J1,2 = 8.0 Hz, 1H, Ar-H), 6.95–6.97 (d, J1,2 = 8.0 Hz, 1H, Ar-H), 7.40–7.44 (td, J1,2 = 4.0 Hz, J1,3 = 8.0 Hz, 1H, Ar-H), 7.85–7.87 (dd, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, Ar-H), 11.19 (s, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 38.55 (C-1), 28.14 (C-2), 80.74 (C-3), 38.01 (C-4), 55.91 (C-5), 18.27 (C-6), 34.31 (C-7), 39.65 (C-8), 49.54 (C-9), 37.37 (C-10), 30.43 (C-11), 75.76 (C-12), 45.08 (C-13), 51.90 (C-14), 30.71 (C-15), 23.70 (C-16), 54.54 (C-17), 15.96 (C-18), 16.66 (C-19), 75.04 (C-20), 19.33 (C-21), 35.38 (C-22), 16.72 (C-23), 37.12 (C-24), 70.79 (C-25), 33.05 (C-26), 26.80 (C-27), 28.52 (C-28), 15.89 (C-29), 17.99 (C-30), 21.43 (–CH3), 171.02 (–C=O); Structure of salicylic acid: 114.06 (C-1), 117.67 (C-3), 118.88 (C-5), 130.30 (C-6), 135.29 (C-4), 161.85 (C-2), 169.76 (C-7, –C=O).
20(R)-Pananxadiol-3β-hydroxy-12β-yl-2′-acetylbenzoate (5b, C39H58O6). White solid, melting point: 226–228 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.74 (s, 3H), 0.77 (s, 3H), 0.85 (s, 3H), 0.98 (s, 6H), 1.00 (s, 3H), 1.01 (s, 3H), 1.11 (s, 3H), 3.17–3.21 (dd, J1,3 = 12.0 Hz, J1,2 = 4.0 Hz, 1H, H-3), 5.13–5.20 (td, J1,3 = 12.0 Hz, J1,2 = 8.0 Hz, 1H, H-12), 7.07–7.09 (d, J = 8.0 Hz, 1H, Ar-H), 7.28–7.31 (t, J1,2 = 6.0 Hz, J1,3 = 12.0 Hz, 1H, Ar-H), 7.50–7.54 (td, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, Ar-H), 8.05–8.08 (dd, J1,2 = 4.0 Hz, J1,3 = 8.0 Hz, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 39.05 (C-1), 28.12 (C-2), 78.95 (C-3), 38.85 (C-4), 55.90 (C-5), 18.39 (C-6), 33.04 (C-7), 39.67 (C-8), 49.78 (C-9), 37.33 (C-10), 30.44 (C-11), 75.53 (C-12), 45.34 (C-13), 52.17 (C-14), 30.88 (C-15), 25.86 (C-16), 53.63 (C-17), 15.90 (C-18), 15.99 (C-19), 75.24 (C-20), 22.94 (C-21), 34.47 (C-22), 16.65 (C-23), 37.23 (C-24), 70.72 (C-25), 32.09 (C-26), 27.40 (C-27), 28.55 (C-28), 15.51 (C-29), 18.29 (C-30); Structure of acetylsalicylic acid: 123.98 (C-8), 124.65 (C-7), 125.89 (C-6), 132.16 (C-5), 133.48 (C-4), 150.87 (C-3), 163.66 (C-2), 169.67 (C-1, C=O), 21.33 (C-9, CH3).
20(R)-Dammarane-20,25-diol-12β-o-acetyl-3β-yl-2′-hydroxybenzoate (6b, C39H60O7). White solid, melting point: 278–280 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.94 (s, 6H), 0.98 (s, 3H), 1.02 (s, 3H), 1.04 (s, 3H), 1.11 (s, 3H),1.22 (s, 6H), 4.74–4.78 (dd, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 2H, H-12, H-3), 6.85–6.89 (t, J1,3 = 16.0 Hz, J1,2 = 8.0 Hz, 1H, Ar-H), 6.97–6.99 (d, J1,2 = 8.0 Hz, 1H, Ar-H), 7.42–7.47 (td, J1,3 = 12.0 Hz, J1,2 = 4.0 Hz, 1H, Ar-H), 7.80–7.83 (dd, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, Ar-H), 10.92 (s, 1H, Ar-OH); 13C-NMR (CDCl3, 100 MHz, ppm): 39.92 (C-1), 28.29 (C-2), 82.08 (C-3), 38.64 (C-4), 56.10 (C-5), 18.10 (C-6), 34.64 (C-7), 42.77 (C-8), 49.16 (C-9), 38.41 (C-10), 31.83 (C-11), 76.64 (C-12), 49.05 (C-13), 52.94 (C-14), 31.72 (C-15), 27.36 (C-16), 50.16 (C-17), 16.42 (C-18), 16.84 (C-19), 73.87 (C-20), 23.16 (C-21), 44.59 (C-22), 18.28 (C-23), 45.66 (C-24), 71.27 (C-25), 29.85 (C-26), 29.60 (C-27), 28.52 (C-28), 15.64 (C-29), 17.78 (C-30), 21.71 (–CH3), 169.94 (–C=O). Structure of salicylic acid: 113.17 (C-1), 117.75 (C-3), 119.18 (C-5), 129.85 (C-6), 135.62 (C-4), 161.89 (C-2), 169.75 (–C=O); MS: m/z 640.89 [M + H]+.
20(R)-Dammarane-20,25-diol-12β-o-acetyl-3β-yl-2′-acetylbenzoate (7b, C39H62O8). White solid, melting point: 268–270 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.91 (s, 6H), 0.96 (s, 6H), 1.02 (s, 3H), 1.10 (s, 3H), 1.21 (s, 6H), 4.68–4.78 (m, 2H, H-12, H-3), 7.08–7.70 (d, J1,2 = 8.0 Hz, 1H, Ar-H), 7.27–7.31 (t, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, Ar-H), 7.51–7.55 (td, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz,1H, Ar-H), 7.95–7.98 (dd, J1,3 =8.0 Hz, J1,2 = 4.0 Hz, 1H, Ar-H); 13C-NMR (CDCl3, 100 MHz, ppm): 38.32 (C-1), 28.19 (C-2), 81.35 (C-3), 38.66 (C-4), 56.12 (C-5), 18.07 (C-6), 34.63 (C-7), 39.87 (C-8), 50.15 (C-9), 37.23 (C-10), 32.05 (C-11), 76.62 (C-12), 49.11 (C-13), 52.89 (C-14), 31.79 (C-15), 27.33 (C-16), 51.35 (C-17), 16.42 (C-18), 16.87 (C-19), 73.85 (C-20), 22.81 (C-21), 44.55 (C-22), 18.24 (C-23), 45.62 (C-24), 71.21 (C-25), 29.56 (C-26), 29.48 (C-27), 29.31 (C-28), 15.60 (C-29), 17.74 (C-30), 21.69 (–CH3), 169.82 (–C=O). Structure of acetylsalicylic acid: 169.72 (C-1, –C=O), 164.02 (C-2), 150.90 (C-3), 133.65 (C-4), 131.41 (C-5), 126.01 (C-6), 124.04 (C-7), 123.88 (C-8), 21.25 (C-9, –CH3). MS: m/z 682.93 [M + H]+.
20(R)-Dammarane-12β,20,25-diol-3β-yl-2′-acetylbenzoate (8b, C37H60O7). White solid, melting point: 254–256 °C, 1H-NMR (CDCl3, 400 MHz, ppm): δ 0.90 (s, 3H), 0.91 (s, 3H), 0.94 (s, 3H), 0.97 (s, 3H), 1.01 (s, 3H), 1.15 (s, 3H), 1.24 (s, 6H), 3.62–3.68 (td, J1,3 = 12.0 Hz, J1,2 = 4.0 Hz, 1H, H-12), 4.68–4.72 (dd, J1,3 = 12.0 Hz,, J1,2 = 8.0 Hz, 1H, H-3), 7.09–7.11 (d, J = 8.0 Hz, 1H, Ar-H), 7.28–7.32 (t, J1,3 = 8.0 Hz, J1,2 = 3.5 Hz, 1H, Ar-H), 7.52–7.56 (td, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H, Ar-H), 7.97–7.99 (dd, J1,3 = 8.0 Hz, J1,2 = 4.0 Hz, 1H); 13C-NMR (CDCl3, 100 MHz, ppm): 39.81 (C-1), 28.23 (C-2), 81.59 (C-3), 38.33 (C-4), 56.17 (C-5), 17.85 (C-6), 34.83 (C-7), 39.91 (C-8), 50.07 (C-9), 37.20 (C-10), 31.36 (C-11), 71.34 (C-12), 48.47 (C-13), 51.78 (C-14), 51.78 (C-15), 31.16 (C-16), 50.13 (C-17), 16.39 (C-18), 16.91 (C-19), 74.80 (C-20), 21.99 (C-21), 42.98 (C-22), 18.30 (C-23), 44.23 (C-24), 71.15 (C-25), 29.56 (C-26), 29.41 (C-27), 28.46 (C-28), 15.81 (C-29), 17.25 (C-30). Structure of acetylsalicylic acid: 169.82 (C-1, –C=O), 164.16 (C-2), 150.89 (C-3), 133.68 (C-4), 131.50 (C-5), 126.07 (C-6), 124.09 (C-7), 123.90 (C-8), 21.30 (C-9, –CH3); MS: m/z 640.89 [M + H]+.