Synthesis of Mesoporous TiO2/Boron-Doped Diamond Photocatalyst and Its Photocatalytic Activity under Deep UV Light (λ = 222 nm) Irradiation
Abstract
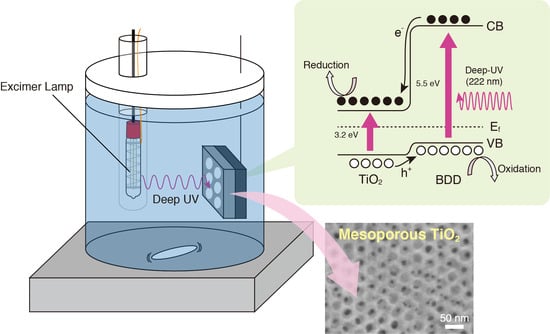
:1. Introduction
2. Results and Discussions
3. Materials and Methods
3.1. Materials
3.2. Synthesis of BDD Layer
3.3. Synthesis of Mesoporous TiO2 Thin Film on BDD Layer
3.4. Characterization of Mesoporous TiO2 Thin Film
3.5. Photocatalytic Activity Test
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suzuki, N.; Athar, T.; Huang, Y.-T.; Shimasaki, K.; Miyamoto, N.; Yamauchi, Y. Synthesis of mesoporous Nb2O5 with crystalline walls and investigation of their photocatalytic activity. J. Ceram. Soc. Jpn. 2011, 119, 405–411. [Google Scholar] [CrossRef]
- Suzuki, N.; Jiang, X.; Radhakrishnan, L.; Takai, K.; Shimasaki, K.; Huang, Y.-T.; Miyamoto, N.; Yamauchi, Y. Hybridization of Photoactive Titania Nanoparticles with Mesoporous Silica Nanoparticles and Investigation of Their Photocatalytic Activity. Bull. Chem. Soc. Jpn. 2011, 84, 812–817. [Google Scholar] [CrossRef]
- Gao, S.; Jiao, S.; Lei, B.; Li, H.; Wang, J.; Yu, Q.; Wang, D.; Guo, F.; Zhao, L. Efficient photocatalyst based on ZnO nanorod arrays/p-type boron-doped-diamond heterojunction. J. Mater. Sci. Mater. Electron. 2015, 26, 1018–1022. [Google Scholar] [CrossRef]
- Yu, H.; Chen, S.; Quan, X.; Zhao, H.; Zhang, Y. Fabrication of a TiO2-BDD Heterojunction and its Application As a Photocatalyst for the Simultaneous Oxidation of an Azo Dye and Reduction of Cr(VI). Environ. Sci. Technol. 2008, 42, 3791–3796. [Google Scholar] [CrossRef]
- Yuan, J.; Li, H.; Gao, S.; Lin, Y.; Li, H. A facile route to n-type TiO2-nanotube/p-type boron-doped-diamond heterojunction for highly efficient photocatalysts. Chem. Commun. 2010, 46, 3119–3121. [Google Scholar] [CrossRef] [PubMed]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, D.; Margolese, D.I.; Chmelka, B.F.; Stucky, G.D. Generalized synthesis of large-pore mesoporous metal oxides with semicrystalline frameworks. Nature 1998, 396, 152–155. [Google Scholar] [CrossRef]
- Jiang, X.; Suzuki, N.; Bastakoti, B.P.; Chen, W.-W.; Huang, Y.-T.; Yamauchi, Y. Controlled Synthesis of Well-Ordered Mesoporous Titania Films with Large Mesopores Templating by Spherical PS-b-PEO Micelles. Eur. J. Inorg. Chem. 2013, 2013, 3286–3291. [Google Scholar] [CrossRef]
- Suzuki, N.; Jiang, X.; Malgras, V.; Yamauchi, Y.; Islam, A.; Han, L. Synthesis of Thin Titania Photoanodes with Large Mesopores for Electricity-generating Windows. Chem. Lett. 2015, 44, 656–658. [Google Scholar] [CrossRef]
- Wu, C.-W.; Ohsuna, T.; Kuwabara, M.; Kuroda, K. Formation of Highly Ordered Mesoporous Titania Films Consisting of Crystalline Nanopillars with Inverse Mesospace by Structural Transformation. J. Am. Chem. Soc. 2006, 128, 4544–4545. [Google Scholar] [CrossRef] [PubMed]
- Oveisi, H.; Jiang, X.; Imura, M.; Nemoto, Y.; Sakamoto, Y.; Yamauchi, Y. A Mesoporous γ-Alumina Film with Vertical Mesoporosity: The Unusual Conversion from a Im3m Mesostructure to Vertically Oriented γ-Alumina Nanowires. Angew. Chem. Int. Ed. 2011, 50, 7410–7413. [Google Scholar] [CrossRef] [PubMed]
- Kidalov, S.V.; Shakhov, F.M. Thermal Conductivity of Diamond Composites. Materials 2009, 2, 2467–2495. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Oyama, T.; Aoshima, A.; Hidaka, H.; Zhao, J.; Serpone, N. Photooxidative N-demethylation of methylene blue in aqueous TiO2 dispersions under UV irradiation. J. Photochem. Photobiol. A-Chem. 2001, 140, 163–172. [Google Scholar] [CrossRef]
- Terashima, C.; Hishinuma, R.; Roy, N.; Sugiyama, Y.; Latthe, S.S.; Nakata, K.; Kondo, T.; Yuasa, M.; Fujishima, A. Charge Separation in TiO2/BDD Heterojunction Thin Film for Enhanced Photoelectrochemical Performance. ACS Appl. Mater. Interfaces 2016, 8, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |







| 222 nm | 308 nm | |
|---|---|---|
| Photolysis | 1.37 × 10−3 | 6.71 × 10−4 |
| TiO2/Glass | 5.22 × 10−3 | 2.70 × 10−3 |
| TiO2/BDD | 7.93 × 10−3 | 1.70 × 10−3 |
| BDD | 5.88 × 10−3 | Does not act as a photocatalyst |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, N.; Okazaki, A.; Kuriyama, H.; Serizawa, I.; Hara, A.; Hirano, Y.; Nakabayashi, Y.; Roy, N.; Terashima, C.; Nakata, K.; et al. Synthesis of Mesoporous TiO2/Boron-Doped Diamond Photocatalyst and Its Photocatalytic Activity under Deep UV Light (λ = 222 nm) Irradiation. Molecules 2018, 23, 3095. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23123095
Suzuki N, Okazaki A, Kuriyama H, Serizawa I, Hara A, Hirano Y, Nakabayashi Y, Roy N, Terashima C, Nakata K, et al. Synthesis of Mesoporous TiO2/Boron-Doped Diamond Photocatalyst and Its Photocatalytic Activity under Deep UV Light (λ = 222 nm) Irradiation. Molecules. 2018; 23(12):3095. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23123095
Chicago/Turabian StyleSuzuki, Norihiro, Akihiro Okazaki, Haruo Kuriyama, Izumi Serizawa, Aiga Hara, Yuiri Hirano, Yukihiro Nakabayashi, Nitish Roy, Chiaki Terashima, Kazuya Nakata, and et al. 2018. "Synthesis of Mesoporous TiO2/Boron-Doped Diamond Photocatalyst and Its Photocatalytic Activity under Deep UV Light (λ = 222 nm) Irradiation" Molecules 23, no. 12: 3095. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23123095