2-(2-Phenylethyl)-4H-chromen-4-one Derivatives from the Resinous Wood of Aquilaria sinensis with Anti-Inflammatory Effects in LPS-Induced Macrophages
Abstract
:1. Introduction
2. Results and Discussion
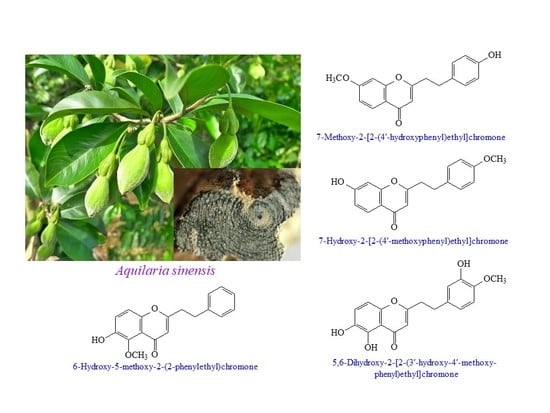
2.1. Isolation and Structural Elucidation
2.2. Structure Identification of the Known Isolates
2.3. Biological Studies
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Biological Assay
3.4.1. Cells and Culture Medium
3.4.2. Luciferase Reporter Assay
3.4.3. Cytotoxicity Assay
3.4.4. Determination of Nitric Oxide (NO) Production
3.4.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2010; pp. 172–173. [Google Scholar]
- Wang, S.L.; Hwang, T.L.; Chung, M.I.; Sung, P.J.; Shu, C.W.; Cheng, M.J.; Chen, J.J. New flavones, a 2-(2-phenylethyl)-4H-chromen-4-one derivative, and anti-inflammatory constituents from the stem barks of Aquilaria sinensis. Molecules 2015, 20, 20912–20925. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.G.; Zhou, M.H.; Lu, J.J.; Yu, B.Y. Antitumor constituents from the leaves of Aquilaria sinensis (Lour.) Gilg. Linchan Huaxue Yu Gongye 2008, 28, 1–5. [Google Scholar]
- Yang, J.S.; Wang, Y.L.; Su, Y.L. Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. IV. Isolation and characterization of 2-(2-phenylethyl)chromone derivatives. Yaoxue Xuebao 1989, 24, 678–683. [Google Scholar]
- Lin, L.D.; Qi, S.Y. Triterpenoid from Chinese eaglewood (Aquilaria sinensis). Zhongcaoyao 2000, 31, 89–90. [Google Scholar]
- Liu, J.; Wu, J.; Zhao, Y.X.; Deng, Y.Y.; Mei, W.L.; Dai, H.F. A new cytotoxic 2-(2-phenylethyl)chromone from Chinese eaglewood. Chin. Chem. Lett. 2008, 19, 934–936. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Z.; Chai, Z.; Zeng, K.; Jia, Y.; Bi, D.; Ma, Z.; Tu, P. Nine 2-(2-phenylethyl)chromone derivatives from the resinous wood of Aquilaria sinensis and their inhibition of LPS-induced NO production in RAW 264.7 cells. Eur. J. Org. Chem. 2012, 27, 5389–5397. [Google Scholar] [CrossRef]
- Yang, D.L.; Wang, H.; Guo, Z.K.; Li, W.; Mei, W.L.; Dai, H.F. Fragrant agarofuran and eremophilane sesquiterpenes in agarwood ‘Qi-Nan’ from Aquilaria sinensis. Phytochem. Lett. 2014, 8, 121–125. [Google Scholar] [CrossRef]
- Jou, I.M.; Lin, C.F.; Tsai, K.J.; Wei, S.J. Macrophage-mediated inflammatory disorders. Mediat. Inflamm. 2013, 2013, 316482. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.L.; Hsu, Y.H.; Lee, P.Y.; Hou, W.C.; Hung, L.C.; Lin, C.H.; Chen, C.M.; Huang, Y.J. Dioscorin isolated from Dioscorea alata activates TLR4-signaling pathways and induces cytokine expression in macrophages. Biochem. Biophys. Res. Commun. 2006, 339, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Chen, S.H.; Lin, L.C.; Fu, S.L. Anti-inflammatory principles from Sarcandra glabra. J. Agric. Food Chem. 2017, 65, 6497–6505. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Tominaga, T.; Konishi, T.; Kiyosawa, S. Studies on the agarwood (Jinko). I. Structures of 2-(2-phenylethyl)chromone derivatives. Chem. Pharm. Bull. 1982, 30, 3791–3795. [Google Scholar] [CrossRef]
- Xia, F.; Sun, J.; Jiang, Y.; Tu, P.F. Further chemical investigation of leaves of Aquilaria Sinensis. Zhongguo Zhongyao Zazhi 2013, 38, 3299–3303. [Google Scholar] [PubMed]
- Riclea, R.; Dickschat, J.S. Identification of intermediates in the biosynthesis of PR toxin by Penicillium roqueforti. Angew. Chem. Int. Ed. 2015, 54, 12167–12170. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Nakahara, S.; Inoue, T.; Sumida, Y.; Takahashi, M.; Masada, Y. A new chromone from agarwood and pyrolysis products of chromone derivatives. Chem. Pharm. Bull. 1985, 33, 5088–5091. [Google Scholar] [CrossRef]
- Ismail, K.A.; Abd El Aziem, T. Synthesis and biological evaluation of some novel 4H-benzopyran-4-one derivatives as nonsteroidal antiestrogens. Eur. J. Med. Chem. 2001, 36, 243–253. [Google Scholar] [CrossRef]
- Iwagoe, K.; Konishi, T.; Kiyosawa, S.; Shimada, Y.; Miyahara, K.; Kawasaki, T. Studies on the agarwood (Jinko). VII. Structures of phenylethylchromone derivatives AH7, AH8 and AH9. Chem. Pharm. Bull. 1988, 36, 2417–2422. [Google Scholar] [CrossRef]
- Konishi, T.; Konoshima, T.; Shimada, Y.; Kiyosawa, S. Six new 2-(2-phenylethyl)chromones from agarwood. Chem. Pharm. Bull. 2002, 50, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Lin, C.H.; Hung, S.K.; Chou, J.H.; Chi, C.W.; Fu, S.L. Fisetin inhibits lipopolysaccharide-induced macrophage activation and dendritic cell maturation. J. Agric. Food Chem. 2010, 58, 10831–10839. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Socolsky, C.; Plietker, B. Total synthesis and absolute configuration assignment of MRSA active garcinol and isogarcinol. Chem.-Eur. J. 2015, 21, 3053–3061. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |








| Compounds a | Relative Luciferase Activity |
|---|---|
| Mean ± SD c | |
| 1 | 0.89 ± 0.05 |
| 2 | 0.93 ± 0.06 |
| 3 | 0.74 ± 0.03 * |
| 4 | 0.92 ± 0.01 |
| 5 | 0.55 ± 0.09 * |
| 6 | 0.75 ± 0.05 * |
| 7 | 0.72 ± 0.16 * |
| 8 | 0.98 ± 0.07 |
| 9 | 1.26 ± 0.40 |
| 10 | 0.54 ± 0.03 * |
| 11 | 0.31 ± 0.05 * |
| 12 | 0.38 ± 0.14 * |
| 13 | 1.09 ± 0.21 |
| LPS-treated vehicle control b | 1.03 ± 0.02 |
| Andrographolide d | 0.35 ± 0.17 * |
| Position | 1 a | 2 a | 3 a | 4 b |
|---|---|---|---|---|
| 3 | 6.07 s | 6.16 s | 6.02 s | 6.06 s |
| 5 | 8.08 d (8.5) | 8.02 d (9.0) | – | – |
| 6 | 6.95 dd (8.5, 2.0) | 7.09 dd (9.0, 2.5) | – | – |
| 7 | – | – | 7.28 d (9.0) | 7.30 d (9.3) |
| 8 | 6.83 d (2.0) | 6.84 d (2.5) | 6.86 d (9.0) | 7.14 d (9.3) |
| 2′ | 7.06 d (8.5) | 7.13 d (8.5) | 6.78 d (2.0) | 7.20 d (7.8) |
| 3′ | 6.76 d (8.5) | 6.83 d (8.5) | – | 7.30 t (7.8) |
| 4′ | – | – | – | 7.22 t (7.8) |
| 5′ | 6.76 d (8.5) | 6.83 d (8.5) | 6.76 d (8.0) | 7.30 t (7.8) |
| 6′ | 7.06 d (8.5) | 7.13 d (8.5) | 6.64 dd (8.0, 2.0) | 7.20 d (7.8) |
| 7′ | 2.98 t (8.0) | 3.05 t (8.0) | 2.96 t (7.0) | 3.04 t (7.8) |
| 8′ | 2.86 t (8.0) | 2.96 t (8.0) | 2.88 t (7.0) | 2.88 t (7.8) |
| OH-5 | – | – | 12.50 s | – |
| OH-6 | – | – | 5.43 s | – |
| OH-3′ | – | – | 5.58 s | – |
| OMe-5 | – | – | – | 3.98 s |
| OMe-7 | 3.91 s | – | – | – |
| OMe-4′ | – | 3.79 | 3.87 s | – |
| Position | 1 a | 2 a | 3 a | 4 b |
|---|---|---|---|---|
| 2 | 168.5 | 168.1 | 170.4 | 167.0 |
| 3 | 109.8 | 110.3 | 108.2 | 110.5 |
| 4 | 177.9 | 177.1 | 183.2 | 177.5 |
| 5 | 127.2 | 125.2 | 145.3 | 143.5 |
| 6 | 116.5 | 113.8 | 140.2 | 146.1 |
| 7 | 164.1 | 156.1 | 121.6 | 120.7 |
| 8 | 100.1 | 103.9 | 110.7 | 114.1 |
| 9 | 158.1 | 156.6 | 144.0 | 151.4 |
| 10 | 115.4 | 118.4 | 111.0 | 117.9 |
| 1′ | 131.5 | 131.5 | 129.5 | 139.8 |
| 2′ | 129.4 | 129.2 | 115.9 | 128.3 |
| 3′ | 115.6 | 114.1 | 144.6 | 128.7 |
| 4′ | 154.5 | 158.3 | 145.9 | 126.6 |
| 5′ | 115.6 | 114.1 | 112.0 | 128.7 |
| 6′ | 129.4 | 129.2 | 123.9 | 128.3 |
| 7′ | 32.0 | 31.9 | 30.3 | 32.9 |
| 8′ | 36.3 | 36.1 | 34.7 | 35.6 |
| OMe-5 | – | – | – | 62.8 |
| OMe-7 | 56.1 | – | – | – |
| OMe-4′ | – | 55.3 | 56.3 | – |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-L.; Tsai, Y.-C.; Fu, S.-L.; Cheng, M.-J.; Chung, M.-I.; Chen, J.-J. 2-(2-Phenylethyl)-4H-chromen-4-one Derivatives from the Resinous Wood of Aquilaria sinensis with Anti-Inflammatory Effects in LPS-Induced Macrophages. Molecules 2018, 23, 289. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23020289
Wang S-L, Tsai Y-C, Fu S-L, Cheng M-J, Chung M-I, Chen J-J. 2-(2-Phenylethyl)-4H-chromen-4-one Derivatives from the Resinous Wood of Aquilaria sinensis with Anti-Inflammatory Effects in LPS-Induced Macrophages. Molecules. 2018; 23(2):289. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23020289
Chicago/Turabian StyleWang, Sin-Ling, Yun-Chen Tsai, Shu-Ling Fu, Ming-Jen Cheng, Mei-Ing Chung, and Jih-Jung Chen. 2018. "2-(2-Phenylethyl)-4H-chromen-4-one Derivatives from the Resinous Wood of Aquilaria sinensis with Anti-Inflammatory Effects in LPS-Induced Macrophages" Molecules 23, no. 2: 289. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23020289