Structure Identification of ViceninII Extracted from Dendrobium officinale and the Reversal of TGF-β1-Induced Epithelial–Mesenchymal Transition in Lung Adenocarcinoma Cells through TGF-β/Smad and PI3K/Akt/mTOR Signaling Pathways
Abstract
:1. Introduction
2. Results
2.1. Structural Identification of ViceninII
2.2. The Cell Viability Effect and Morphology Changes of ViceninII and Transforming Growth Factor 1 (TGF-β1) on Lung Adenocarcinoma A549 and H1299 Cells
2.3. Clonogenic Formation in Human Lung Adenocarcinoma A549 and H1299 Cells
2.4. ViceninII Suppressed TGF-β1-Induced Migration and Invasion in A549 and H1299 Cells
2.5. ViceninII Reversed TGF-β1-Induced Expression of EMT Biomarkers in A549 and H1299 Cells
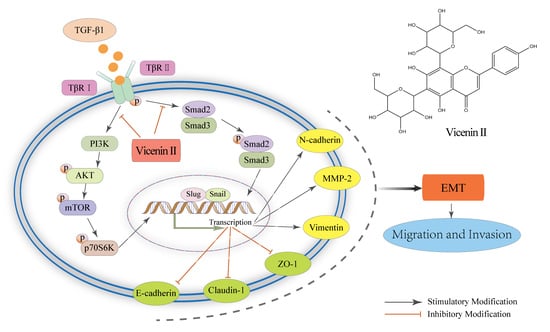
2.6. The Inhibitory Effect of ViceninII on TGF-β1-Induced EMT in Lung Cancer A549 and H1299 Cells through the TGF-β/Smad and PI3K/Akt/mTOR Pathways
3. Discussion
4. Materials and Methods
4.1. Plant Material and Reagents
4.2. Extraction and Isolation
4.3. Structural Analysis of ViceninII
4.4. Cell Culture and Drug Treatment
4.5. Effect of ViceninII and TGF-β1 on Cell Viability
4.6. Morphology Observations
4.7. Colony Forming Assay
4.8. Wound Healing Migration Assay
4.9. Transwell Invasion Assay
4.10. Immunofluorescence Assay
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| UV | Ultraviolet Radiation |
| HPLC | High-Performance Liquid Chromatography |
| LC-MS | Liquid Chromatography-Mass spectrometry |
| NMR | Nuclear Magnetic Resonance |
| ODS | Octadecyl silica |
| TGF-β1 | Transforming Growth Factor-β1 |
| EMT | Epithelial–Mesenchymal Transition |
| MMPs | Matrix Metalloproteinases |
| PI3K | Phosphatidylinositol-3-Kinase |
| Akt | Protein Kinase B |
| P70S6K | p70 Ribosomal Protein S6 Kinase |
| mTOR | Mammalian Target of Rapamycin |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Fedor, D.; Johnson, W.R.; Singhal, S. Local recurrence following lung cancer surgery: Incidence, risk factors, and outcomes. Surg. Oncol. 2013, 22, 156–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Zheng, C.; Xu, H.; He, W.; Ruan, Y.; Ma, J.; Zheng, J.; Ye, C.; Li, W. Inhibition of AMPK-related kinase 5 (ARK5) enhances cisplatin cytotoxicity in non-small cell lung cancer cells through regulation of epithelial-mesenchymal transition. Am. J. Transl. Res. 2017, 9, 1708–1719. [Google Scholar] [PubMed]
- Barton, M.K. Patients of all ages with advanced non-small cell lung cancer are not receiving chemotherapy. CA Cancer J. Clin. 2015, 65, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.G.; Haines, C.; Perkel, R.; Enck, R.E. Lung cancer: Diagnosis and management. Am. Fam. Phys. 2007, 75, 56–63. [Google Scholar]
- Tsai, J.H.; Yang, J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, W.J.; Kim, H.; Park, K.K. The biological role of epithelial-mesenchymal transition in lung cancer (Review). Oncol. Rep. 2016, 36, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Chockley, P.J.; Chen, J.; Chen, G.; Beer, D.G.; Standiford, T.J.; Keshamouni, V.G. Epithelial-mesenchymal transition leads to NK cell-mediated metastasis-specific immunosurveillance in lung cancer. J. Clin. Investig. 2018, 128, 1384–1396. [Google Scholar] [CrossRef]
- De Craene, B.; Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nature reviews. Cancer 2013, 13, 97–110. [Google Scholar]
- Yang, D.; Cao, X.; Wang, F.; Jiang, H.; Feng, D.; Guo, H.; Du, L.; Jin, Y.; Chen, Y.; Yin, X.; et al. LFG-500, a novel synthetic flavonoid, suppresses epithelial-mesenchymal transition in human lung adenocarcinoma cells by inhibiting NLRP3 in inflammatory microenvironment. Cancer Lett. 2017, 400, 137–148. [Google Scholar] [CrossRef]
- Da, C.; Liu, Y.; Zhan, Y.; Liu, K.; Wang, R. Nobiletin inhibits epithelial-mesenchymal transition of human non-small cell lung cancer cells by antagonizing the TGF-β/Smad3 signaling pathway. Oncol. Rep. 2016, 35, 2767–2774. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Lu, J.J.; Wang, Y.; Pei, L.; Chen, X. Osthole inhibited TGF β-induced epithelial-mesenchymal transition (EMT) by suppressing NF-κB mediated Snail activation in lung cancer A549 cells. Cell Adhes. Migr. 2017, 11, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Schmalfeldt, B.; Prechtel, D.; Harting, K.; Spathe, K.; Rutke, S.; Konik, E.; Fridman, R.; Berger, U.; Schmitt, M.; Kuhn, W.; et al. Increased expression of matrix metalloproteinases (MMP)-2, MMP-9, and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin. Cancer Res. 2001, 7, 2396–2404. [Google Scholar] [PubMed]
- Inukai, T.; Inoue, A.; Kurosawa, H.; Goi, K.; Shinjyo, T.; Ozawa, K.; Mao, M.; Inaba, T.; Look, A.T. SLUG, a ces-1-related zinc finger transcription factor gene with antiapoptotic activity, is a downstream target of the E2A-HLF oncoprotein. Mol. Cell 1999, 4, 343–352. [Google Scholar] [CrossRef]
- Bolos, V.; Peinado, H.; Perez-Moreno, M.A.; Fraga, M.F.; Esteller, M.; Cano, A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: A comparison with Snail and E47 repressors. J. Cell Sci. 2003, 116, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.; Park, S.J.; Choi, Y.S.; Jeon, W.K.; Kim, B.C. Kaempferol Suppresses Transforming Growth Factor-β1-Induced Epithelial-to-Mesenchymal Transition and Migration of A549 Lung Cancer Cells by Inhibiting Akt1-Mediated Phosphorylation of Smad3 at Threonine-179. Neoplasia 2015, 17, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Ikushima, H.; Miyazono, K. TGFβ signalling: A complex web in cancer progression. Nat. Rev. Cancer 2010, 10, 415–424. [Google Scholar] [CrossRef]
- Lin, C.Y.; Hsieh, Y.H.; Yang, S.F.; Chu, S.C.; Chen, P.N.; Hsieh, Y.S. Cinnamomum cassia extracts reverses TGF-β1-induced epithelial-mesenchymal transition in human lung adenocarcinoma cells and suppresses tumor growth in vivo. Environ. Toxicol. 2017, 32, 1878–1887. [Google Scholar] [CrossRef]
- Smith, A.L.; Robin, T.P.; Ford, H.L. Molecular pathways: Targeting the TGF-β pathway for cancer therapy. Clin. Cancer Res. 2012, 18, 4514–4521. [Google Scholar] [CrossRef]
- Massague, J.; Seoane, J.; Wotton, D. Smad transcription factors. Genes Dev. 2005, 19, 2783–2810. [Google Scholar] [CrossRef] [Green Version]
- Bakin, A.V.; Tomlinson, A.K.; Bhowmick, N.A.; Moses, H.L.; Arteaga, C.L. Phosphatidylinositol 3-kinase function is required for transforming growth factor β-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 2000, 275, 36803–36810. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Ko, J.H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.G.; Yang, W.M.; Um, J.Y.; et al. Ginkgolic Acid Inhibits Invasion and Migration and TGF-β-Induced EMT of Lung Cancer Cells through PI3K/Akt/mTOR Inactivation. J. Cell. Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.; Becerril, C.; Montano, M.; Garcia-De-Alba, C.; Ramirez, R.; Checa, M.; Pardo, A.; Selman, M. FGF-1 reverts epithelial-mesenchymal transition induced by TGF-{β}1 through MAPK/ERK kinase pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, L222–L231. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.A.; Ghiassi, M.; Bakin, A.; Aakre, M.; Lundquist, C.A.; Engel, M.E.; Arteaga, C.L.; Moses, H.L. Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell 2001, 12, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Zhang, Z.; Jiang, B.H.; Shi, X. Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochem. Biophys. Res. Commun. 2003, 310, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- LoPiccolo, J.; Blumenthal, G.M.; Bernstein, W.B.; Dennis, P.A. Targeting the PI3K/Akt/mTOR pathway: Effective combinations and clinical considerations. Drug Resist. Updat. 2008, 11, 32–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulpitt, C.J.; Li, Y.; Bulpitt, P.F.; Wang, J. The use of orchids in Chinese medicine. J. R. Soc. Med. 2007, 100, 558–563. [Google Scholar] [CrossRef]
- Ng, T.B.; Liu, J.; Wong, J.H.; Ye, X.; Wing Sze, S.C.; Tong, Y.; Zhang, K.Y. Review of research on Dendrobium, a prized folk medicine. Appl. Microbiol. Biotechnol. 2012, 93, 1795–1803. [Google Scholar] [CrossRef]
- Li, X.X.; Ding, X.Y.; Chu, B.H.; Zhou, Q.; Ding, G.; Gu, S. Genetic diversity analysis and conservation of the endangered Chinese endemic herb Dendrobium officinale Kimura et Migo (Orchidaceae) based on AFLP. Genetica 2008, 133, 159–166. [Google Scholar] [CrossRef]
- Liu, X.F.; Zhu, J.; Ge, S.Y.; Xia, L.J.; Yang, H.Y.; Qian, Y.T.; Ren, F.Z. Orally administered Dendrobium officinale and its polysaccharides enhance immune functions in BALB/c mice. Nat. Prod. Commun. 2011, 6, 867–870. [Google Scholar]
- Cai, H.; Huang, X.; Nie, S.; Xie, M.; Phillips, G.O.; Cui, S.W. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan®): Part III–Immunomodulatory activity in vitro. Bioact. Carbohydr. Dietary Fibre 2015, 5, 99–105. [Google Scholar] [CrossRef]
- Huang, K.W.; Li, Y.R.; Tao, S.C.; Wei, G.; Huang, Y.C.; Chen, D.F.; Wu, C.F. Purification, Characterization and Biological Activity of Polysaccharides from Dendrobium officinale. Molecules 2016, 21, 701. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.J.; Nie, S.P.; Cai, H.L.; Zhang, G.Y.; Cui, S.W.; Xie, M.Y.; Phillips, G.O. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan®): Part VI. Protective effects against oxidative stress in immunosuppressed mice. Food Res. Int. 2015, 72, 168–173. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, J.; Han, J.; Shu, H.; Liu, K. Isolation of polysaccharides from Dendrobium officinale leaves and anti-inflammatory activity in LPS-stimulated THP-1 cells. Chem. Cent. J. 2018, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Ren, Z.; Zhang, X.; Xing, S.; Tao, S.; Liu, C.; Wei, G.; Yuan, Y.; Lei, Z. Structural Characterization of Polysaccharides from Dendrobium officinale and Their Effects on Apoptosis of HeLa Cell Line. Molecules 2018, 23, 2484. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Zhang, X.; Ke, H.; Lin, J.; Huang, Y.; Wei, G. Physicochemical properties of polysaccharides from Dendrobium officinale by fractional precipitation and their preliminary antioxidant and anti-HepG2 cells activities in vitro. Chem. Cent. J. 2018, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Luo, D.; Liu, J.; Wu, X.; Chen, J.; Huang, Q.; Su, L.; Zeng, L.; Wang, H.; Su, Z. Hepatoprotective Effect of Polysaccharides Isolated from Dendrobium officinale against Acetaminophen-Induced Liver Injury in Mice via Regulation of the Nrf2-Keap1 Signaling Pathway. Oxidat. Med. Cell. Longevity 2018, 2018, 6962439. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Xie, Z.; Lei, Z.; Huang, Y.; Wei, G. Simultaneous identification and determination of flavonoids in Dendrobium officinale. Chem. Cent. J. 2018, 12, 40. [Google Scholar] [CrossRef]
- Xing, S.; Yu, W.; Zhang, X.; Luo, Y.; Lei, Z.; Huang, D.; Lin, J.; Huang, Y.; Huang, S.; Nong, F.; et al. Isoviolanthin Extracted from Dendrobium officinale Reverses TGF-β1-Mediated Epithelial(-)Mesenchymal Transition in Hepatocellular Carcinoma Cells via Deactivating the TGF-β/Smad and PI3K/Akt/mTOR Signaling Pathways. Int. J. Mol. Sci. 2018, 19, 1556. [Google Scholar] [CrossRef]
- Gouvea, D.R.; de Barros Bello Ribeiro, A.; Thormann, U.; Lopes, N.P.; Butterweck, V. Evaluation of intestinal permeability of vicenin-2 and lychnopholic acid from Lychnophora salicifolia (Brazilian arnicao) using Caco-2 cells. J. Nat. Prod. 2014, 77, 464–471. [Google Scholar] [CrossRef]
- Silva, D.B.; Turatti, I.C.; Gouveia, D.R.; Ernst, M.; Teixeira, S.P.; Lopes, N.P. Mass spectrometry of flavonoid vicenin-2, based sunlight barriers in Lychnophora species. Sci. Rep. 2014, 4, 4309. [Google Scholar] [CrossRef]
- Tao, Y.; Cai, H.; Li, W.D.; Cai, B.C. Ultrafiltration coupled with high-performance liquid chromatography and quadrupole-time-of-flight mass spectrometry for screening lipase binders from different extracts of Dendrobium officinale. Anal. Bioanal. Chem. 2015, 407, 6081–6093. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, S.L.; Tomaz, J.C.; Lopes, N.P. Liquid chromatography-tandem mass spectrometric method for determination of the anti-inflammatory compound vicenin-2 in the leaves of L. ericoides Mart. Biomed. Chromatogr. BMC 2007, 21, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Bae, J.S. Vicenin-2 and scolymoside inhibit high-glucose-induced vascular inflammation in vitro and in vivo. Can. J. Physiol. Pharmacol. 2016, 94, 287–295. [Google Scholar] [CrossRef]
- Kang, H.; Ku, S.K.; Jung, B.; Bae, J.S. Anti-inflammatory effects of vicenin-2 and scolymoside in vitro and in vivo. Inflamm. Res. 2015, 64, 1005–1021. [Google Scholar] [CrossRef] [PubMed]
- Marrassini, C.; Davicino, R.; Acevedo, C.; Anesini, C.; Gorzalczany, S.; Ferraro, G. Vicenin-2, a potential anti-inflammatory constituent of Urtica circularis. J. Nat. Prod. 2011, 74, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Ishita, I.J.; Jung, H.A.; Choi, J.S. Vicenin 2 isolated from Artemisia capillaris exhibited potent anti-glycation properties. Food Chem. Toxicol. 2014, 69, 55–62. [Google Scholar] [CrossRef]
- Verspohl, E.J.; Fujii, H.; Homma, K.; Buchwald-Werner, S. Testing of Perilla frutescens extract and Vicenin 2 for their antispasmodic effect. Phytomed. Int. J. Phytother. Phytopharmacol. 2013, 20, 427–431. [Google Scholar] [CrossRef]
- Lee, W.; Yoon, E.K.; Kim, K.M.; Park, D.H.; Bae, J.S. Antiseptic effect of vicenin-2 and scolymoside from Cyclopia subternata (honeybush) in response to HMGB1 as a late sepsis mediator in vitro and in vivo. Can. J. Physiol. Pharmacol. 2015, 93, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Bae, J.S. Antithrombotic and antiplatelet activities of vicenin-2. Blood Coagul. Fibrinol. 2015, 26, 628–634. [Google Scholar] [CrossRef]
- Nagaprashantha, L.D.; Vatsyayan, R.; Singhal, J.; Fast, S.; Roby, R.; Awasthi, S.; Singhal, S.S. Anti-cancer effects of novel flavonoid vicenin-2 as a single agent and in synergistic combination with docetaxel in prostate cancer. Biochem. Pharmacol. 2011, 82, 1100–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhal, S.S.; Jain, D.; Singhal, P.; Awasthi, S.; Singhal, J.; Horne, D. Targeting the mercapturic acid pathway and vicenin-2 for prevention of prostate cancer. Biochim. Biophys. Acta—Rev. Cancer 2017, 1868, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, X.; Zhang, W.; Rengarajan, T. Vicenin-2 inhibits Wnt/β-catenin signaling and induces apoptosis in HT-29 human colon cancer cell line. Drug Des. Dev. Ther. 2018, 12, 1303–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.; Ku, S.K.; Bae, J.S. Ameliorative Effect of Vicenin-2 and Scolymoside on TGFBIp-Induced Septic Responses. Inflammation 2015, 38, 2166–2177. [Google Scholar] [CrossRef]
- Baruah, T.J.; Kma, L. Vicenin-2 acts as a radiosensitizer of the non-small cell lung cancer by lowering Akt expression. BioFactors 2018. [Google Scholar] [CrossRef] [PubMed]
- Velozo, L.S.; Ferreira, M.J.; Santos, M.I.; Moreira, D.L.; Guimaraes, E.F.; Emerenciano, V.P.; Kaplan, M.A. C-glycosyl flavones from Peperomia blanda. Fitoterapia 2009, 80, 119–122. [Google Scholar] [CrossRef]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wan, L.; Liu, Z.; Xu, G.; Wang, S.; Su, Z.; Zhang, Y.; Zhang, C.; Liu, X.; Lei, Z.; et al. Long non-coding RNA XIST promotes TGF-β-induced epithelial-mesenchymal transition by regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer. Cancer Lett. 2018, 418, 185–195. [Google Scholar] [CrossRef]
- Levi-Schaffer, F.; Garbuzenko, E.; Rubin, A.; Reich, R.; Pickholz, D.; Gillery, P.; Emonard, H.; Nagler, A.; Maquart, F.A. Human eosinophils regulate human lung- and skin-derived fibroblast properties in vitro: A role for transforming growth factor β (TGF-β). Proc. Natl. Acad. Sci. USA 1999, 96, 9660–9665. [Google Scholar] [CrossRef]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Aiello, N.M.; Brabletz, T.; Kang, Y.; Nieto, M.A.; Weinberg, R.A.; Stanger, B.Z. Upholding a role for EMT in pancreatic cancer metastasis. Nature 2017, 547, E7–E8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masilamani, T.J.; Rintala-Maki, N.D.; Wang, K.; Sutherland, L.C. Downregulating activated epidermal growth factor receptor has no effect on RBM5 expression. Chin. Med. J. 2012, 125, 2378–2381. [Google Scholar] [PubMed]
- Wang, H.; Zhao, Y.; Cao, L.; Zhang, J.; Wang, Y.; Xu, M. Metastasis suppressor protein 1 regulated by PTEN suppresses invasion, migration, and EMT of gastric carcinoma by inactivating PI3K/AKT signaling. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, B.; Xiu, Z.; Zhou, Z.; Liu, J.; Li, X.; Tang, X. PI3K/Akt/HIF-1alpha signaling pathway mediates HPV-16 oncoprotein-induced expression of EMT-related transcription factors in non-small cell lung cancer cells. J. Cancer 2018, 9, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, P.; Shao, M.; Zang, X.; Zhang, J.; Mao, F.; Qian, H.; Xu, W. SALL4 activates TGF-β/SMAD signaling pathway to induce EMT and promote gastric cancer metastasis. Cancer Manag. Res. 2018, 10, 4459–4470. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, C.J.; Matter, W.F.; Hui, K.Y.; Brown, R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biolog. Chem. 1994, 269, 5241–5248. [Google Scholar]
- Laping, N.J.; Grygielko, E.; Mathur, A.; Butter, S.; Bomberger, J.; Tweed, C.; Martin, W.; Fornwald, J.; Lehr, R.; Harling, J.; et al. Inhibition of transforming growth factor (TGF)-β1-induced extracellular matrix with a novel inhibitor of the TGF-β type I receptor kinase activity: SB-431542. Mol. Pharmacol. 2002, 62, 58–64. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound ViceninII and Dendrobium officinale are available from the corresponding author Gang Wei. |







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Ren, Z.; Du, B.; Xing, S.; Huang, S.; Li, Y.; Lei, Z.; Li, D.; Chen, H.; Huang, Y.; et al. Structure Identification of ViceninII Extracted from Dendrobium officinale and the Reversal of TGF-β1-Induced Epithelial–Mesenchymal Transition in Lung Adenocarcinoma Cells through TGF-β/Smad and PI3K/Akt/mTOR Signaling Pathways. Molecules 2019, 24, 144. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24010144
Luo Y, Ren Z, Du B, Xing S, Huang S, Li Y, Lei Z, Li D, Chen H, Huang Y, et al. Structure Identification of ViceninII Extracted from Dendrobium officinale and the Reversal of TGF-β1-Induced Epithelial–Mesenchymal Transition in Lung Adenocarcinoma Cells through TGF-β/Smad and PI3K/Akt/mTOR Signaling Pathways. Molecules. 2019; 24(1):144. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24010144
Chicago/Turabian StyleLuo, Yingyi, Zhiyao Ren, Biaoyan Du, Shangping Xing, Shaowei Huang, Yunrong Li, Zhouxi Lei, Dan Li, Huanhuan Chen, Yuechun Huang, and et al. 2019. "Structure Identification of ViceninII Extracted from Dendrobium officinale and the Reversal of TGF-β1-Induced Epithelial–Mesenchymal Transition in Lung Adenocarcinoma Cells through TGF-β/Smad and PI3K/Akt/mTOR Signaling Pathways" Molecules 24, no. 1: 144. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24010144