Effects of β-glucosidase and α-rhamnosidase on the Contents of Flavonoids, Ginkgolides, and Aroma Components in Ginkgo Tea Drink
Abstract
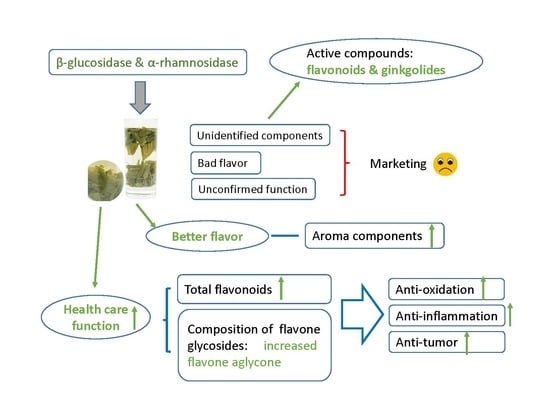
:1. Introduction
2. Results and Discussion
2.1. Ginkgo Tea Can Be Brewed Repeatedly for at Least Three Times Considering the Release of Flavonoids and Ginkgolides
2.2. The Addition of Glycosidase When Making the Tea Promotes the Release of Flavonoids and Aroma Components from the Ginkgo Tea
2.3. The Addition of Glycosidase during the Making of the Tea Increases the Content of Flavone Aglycone in the Tea Infusion
2.4. The Addition of Glycosidase during the Making of the Tea Promotes the Health Care Function of Ginkgo Tea
3. Materials and Methods
3.1. Materials
3.2. Animals
3.3. Cell Culture
3.4. Enzyme Preparation
3.5. Making Ginkgo Tea
3.6. Extraction of Total Ginkgo Flavonoids in Ginkgo Tea and the Residue of Ginkgo Tea
3.7. Analysis of Ginkgo Flavonoids
3.8. Extraction of Ginkgolides in Ginkgo Tea and the Residue of Ginkgo Tea
3.9. Analysis of Ginkgolides
3.10. Extraction and Analysis of Aroma Components
3.11. Enzyme Treatment of Ginkgo Tea
3.12. ABTS Radical Scavenging Activity
3.13. Free Radical (DPPH) Scavenging Experiment
3.14. Lymphocyte Transformation
3.15. MTT Assay
3.16. Determination of NO Production by Griess Method
3.17. In Vitro Proliferation Experiment
3.18. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, C.Y.; Yang, J.J.; Lee, S.S.; Chen, C.J.; Huang, Y.C.; Huang, K.H.; Kuan, Y.H. Protective effect of Ginkgo biloba leaves extract, EGb761, on endotoxin-induced acute lung injury via a JNK- and Akt-dependent NFkappaB pathway. J. Agric. Food Chem. 2014, 62, 6337–6344. [Google Scholar] [CrossRef]
- Li, L.; Xie, S.; Ning, J.; Chen, Q.; Zhang, Z. Evaluating green tea quality based on multisensor data fusion combining hyperspectral imaging and olfactory visualization systems. J. Sci. Food Agric. 2019, 99, 1787–1794. [Google Scholar] [CrossRef]
- Nagai, E.; Iwai, M.; Koketsu, R.; Sogabe, R.; Morimoto, R.; Suzuki, Y.; Ohta, Y.; Okuno, Y.; Ohshima, A.; Enomoto, T.; et al. Inhibition of influenza virus replication by adlay tea. J. Sci. Food. Agric. 2017, 98, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.Z.; Ren, J.M.; Li, Y.L.; Yin, H. Process optimization of Ginkgo biloba tea artificial inoculation fermentation. Food Sci. Technol. 2014, 39, 114–117. [Google Scholar]
- Zeng, X. Bioactive Compounds of Ginkgo Biloba leaves and Application to Functional Foods. Sci. Technol. West. China 2007, 12, 22–26. [Google Scholar]
- Zhang, H.; Li, Y.; Lv, Y.; Jiang, Y.; Pan, J.; Duan, Y.; Zhu, Y.; Zhang, S. Influence of brewing conditions on taste components in Fuding white tea infusions. J. Sci. Food Agric. 2017, 97, 2826–2833. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, Y.; Shi, Y.; He, X.; Chen, X. Impact of elevated CO2 and O3 concentrations on biogenic volatile organic compounds emissions from Ginkgo biloba. Bull Environ. Contam. Toxicol. 2009, 82, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Liao, D.; Zhang, Y. Chemical studies on volatile constituents of Ginkgo biloba L. leaves. Nat. Prod. Res. Dev. 1999, 11, 62–66. [Google Scholar]
- Kakigi, Y.; Hakamatsuka, T.; Icho, T.; Goda, Y.; Mochizuki, N. Comprehensive Analysis of Flavonols in Ginkgo biloba Products by Ultra-High-Performance Liquid Chromatography Coupled with Ultra-Violet Detection and Time-of-Flight Mass Spectrometry. Biosci. Biotechnol. Biochem. 2012, 76, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, J.-K.; Yan, R.; Li, L.; Xiao, Z.-Y.; Zhou, W.-X.; Wang, Z.-Z.; Xiao, W.; Du, G.-H. Diterpene ginkgolides protect against cerebral ischemia/reperfusion damage in rats by activating Nrf2 and CREB through PI3K/Akt signaling. Acta Pharmacol. Sin. 2018, 39, 1259–1272. [Google Scholar] [CrossRef]
- Zhou, G.; Ma, J.; Tang, Y.; Wang, X.; Zhang, J.; Duan, J.-A.; Santos-Buelga, C.; McPhee, D.J. Multi-Response Optimization of Ultrasonic Assisted Enzymatic Extraction Followed by Macroporous Resin Purification for Maximal Recovery of Flavonoids and Ginkgolides from Waste Ginkgo biloba Fallen Leaves. Molecules 2018, 23, 1029. [Google Scholar] [CrossRef]
- Song, Y.-R.; Sung, S.-K.; Jang, M.; Lim, T.-G.; Cho, C.-W.; Han, C.-J.; Hong, H.-D. Enzyme-assisted extraction, chemical characteristics, and immunostimulatory activity of polysaccharides from Korean ginseng (Panax ginseng Meyer). Int. J. Boil. Macromol. 2018, 116, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-G.; Lu, Y.; Wang, W.-N.; Liu, Z.-Y.; Liu, J.-W.; Chen, X.-Q. A novel enzyme-assisted approach for efficient extraction of Z-ligustilide from Angelica sinensis plants. Sci. Rep. 2017, 7, 9783. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Sousa, S.; Silva, A.; Amorim, M.; Pereira, L.; Rocha-Santos, T.A.P.; Gomes, A.M.P.; Duarte, A.C.; Freitas, A.C. Impact of Enzyme- and Ultrasound-Assisted Extraction Methods on Biological Properties of Red, Brown, and Green Seaweeds from the Central West Coast of Portugal. J. Agric. Food Chem. 2015, 63, 3177–3188. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.B.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef]
- Lu, Q.; Hao, M.; Wu, W.; Zhang, N.; Isaac, A.T.; Yin, J.; Zhu, X.; Du, L.; Yin, X. Antidiabetic cataract effects of GbE, rutin and quercetin are mediated by the inhibition of oxidative stress and polyol pathway. Acta Biochim. Pol. 2018, 65, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, M.; Xu, G.; Tian, Y.; Tang, H.; Wang, Y. Metabolomics Reveals that Dietary Ferulic Acid and Quercetin Modulate Metabolic Homeostasis in Rats. J. Agric. Food Chem. 2018, 66, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Wu, Q.; Yao, X.; Cheng, Z. Rapid and Sensitive Determination of Major Active Ingredients and Toxic Components in Ginkgo Biloba Leaves Extract (EGb 761) by a Validated UPLC–MS-MS Method. J. Chromatogr. Sci. 2017, 55, 459–464. [Google Scholar]
- Pei, J.; Chen, A.; Zhao, L.; Cao, F.; Ding, G.; Xiao, W. One-Pot Synthesis of Hyperoside by a Three-Enzyme Cascade Using a UDP-Galactose Regeneration System. J. Agric. Food Chem. 2017, 65, 6042–6048. [Google Scholar] [CrossRef]
- Ge, L.; Xie, J.; Wu, T.; Zhang, S.; Zhao, L.; Ding, G.; Wang, Z.; Xiao, W. Purification and characterisation of a novel α-l-rhamnosidase exhibiting transglycosylating activity from Aspergillus oryzae. Int. J. Food Sci. Tech. 2017, 52, 2596–2603. [Google Scholar] [CrossRef]
- Zhou, J.L.; Fang, X.Y.; Wang, J.Q.; Zhao, L.G.; Li, Y.; Tang, F.; Yue, Y.D. Structures and bioactivities of seven flavonoids from Osmanthus fragrans ‘Jinqiu’ essential oil extraction residues. Nat. Prod. Res. 2018, 32, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Xie, J.; Yin, R.; Zhao, L.; Ding, G.; Wang, Z.; Xiao, W. Enzymatic transformation of ginsenoside Rb1 to ginsenoside 20(S)-Rg3 by GH3 β-glucosidase from Thermotoga thermarum DSM 5069T. J. Mol. Catal. B-Enzym. 2015, 113, 104–109. [Google Scholar] [CrossRef]
- Xie, J.C.; Zhao, D.X.; Zhao, L.G.; Pei, J.J.; Xiao, W.; Ding, G.; Wang, Z.Z. Overexpression and characterization of a Ca2+ activated thermostable β-glucosidase with high ginsenoside Rb1 to ginsenoside 20(S)-Rg3 bioconversion productivity. J. Ind. Microbiol. Biotechnol. 2015, 42, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Chen, A.; Pei, J.; Zhao, L.; Fang, X.; Ding, G.; Wang, Z.; Xiao, W.; Tang, F. Enhancing the thermostability of α-L-rhamnosidase from Aspergillus terreus and the enzymatic conversion of rutin to isoquercitrin by adding sorbitol. BMC Biotechnol. 2017, 17, 10886. [Google Scholar] [CrossRef]
- Fransen, H.P.; Pelgrom, S.M.; Stewart-Knox, B.; De Kaste, D.; Verhagen, H. Assessment of health claims, content, and safety of herbal supplements containing Ginkgo biloba. Food Nutr. Res. 2010, 54, 5221. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity12. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Lin, S.; Ye, J.; Zhang, W.-D.; Cao, B.-J.; Xu, X.-K.; Shan, L.; Su, J. Development and Validation of an Analytical Method for the Determination of Flavonol Glycosides in Ginkgo Leaves and ShuXueNing Injections by a Single Marker. J. Chromatogr. Sci. 2016, 54, 1041–1049. [Google Scholar] [CrossRef]
- Zuo, W.; Yan, F.; Zhang, B.; Li, J.; Mei, D.; Zuo, F.Y.W. Advances in the Studies of Ginkgo Biloba Leaves Extract on Aging-Related Diseases. Aging Disease 2017, 8, 812–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Li, Y.; Wang, J.; Sun, K.; Tao, W.; Wang, Z.; Xiao, W.; Pan, Y.; Zhang, S.; Wang, Y. Systematic Investigation of Ginkgo Biloba Leaves for Treating Cardio-cerebrovascular Diseases in an Animal Model. ACS Chem. Boil. 2017, 12, 1363–1372. [Google Scholar] [CrossRef]
- Majd, S.; Power, J.H. Oxidative Stress and Decreased Mitochondrial Superoxide Dismutase 2 and Peroxiredoxins 1 and 4 Based Mechanism of Concurrent Activation of AMPK and mTOR in Alzheimer’s Disease. Curr. Alzheimer Res. 2018, 15, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Kawamura, M.; Yukami, T.; Ishihara, M.; Bamba, Y.; Kaneshiro, S.; Tsuboi, H.; Yamamoto, K. Etiology of acute ischemic cerebrovascular disease associated with rheumatoid arthritis: Changes with progression of anti-inflammatory therapy. Eur. J. Neurol. 2018, 25, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Daglia, M.; Braidy, N.; Loizzo, M.; Tundis, R.; Nabavi, S. Neuroprotective Effects of Ginkgolide B Against Ischemic Stroke: A Review of Current Literature. Top. Med. Chem. 2015, 15, 2222–2232. [Google Scholar]
- Isah, T. Rethinking Ginkgo biloba L.: Medicinal uses and conservation. Pharmacogn. Rev. 2015, 9, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Wu, T.; Xiao, W.; Wang, Z.Z.; Ding, G.; Zhao, L.G. Enrichment and purification of total ginkgo flavonoid O-glycosides from Ginkgo biloba extract with macroporous resin and evaluation of anti-inflammation activities in vitro. Molecules 2018, 23, E1167. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Jiang, Y.; Ji, H.; Zhao, L.; Xiao, W.; Wang, Z.; Ding, G. The Synergistic Beneficial Effects of Ginkgo Flavonoid and Coriolus versicolor Polysaccharide for Memory Improvements in a Mouse Model of Dementia. Evid. Based Complement Alternat. Med. 2015, 2015, 128394. [Google Scholar]
- Zhou, T.; Fan, S.; You, L.; Zhao, L.; Li, X. Expression and characterization of GH3 β-Glucosidase from Aspergillus niger NL-1 with high specific activity, glucose inhibition and solvent tolerance. Microbiology 2013, 82, 356–363. [Google Scholar] [CrossRef]
- Zhao, L.G.; Pei, J.J.; Feng, Y.Y.; Tang, F.; Yue, Y.D.; Cao, H.Q.; Yu, X.D. α-L-rhamnosidase Rha1, The Expression Genes and Applications. Patent CN104312996B, 22 March 2017. [Google Scholar]
- Wu, T.; Pei, J.; Ge, L.; Wang, Z.; Ding, G.; Xiao, W.; Zhao, L. Characterization of a α-l-rhamnosidase from Bacteroides thetaiotaomicron with high catalytic efficiency of epimedin C. Bioorganic Chem. 2018, 81, 461–467. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 1st ed.; China Medical Science Press: Beijing, China, 2015; Volume 1, p. 316. [Google Scholar]
- Long, S.; Resende, D.I.S.P.; Kijjoa, A.; Silva, A.M.S.; Fernandes, R.; Xavier, C.P.R.; Vasconcelos, M.H.; Sousa, E.; Pinto, M.M.M. Synthesis of New proteomimetic quinazolinone alkaloids and evaluation of their neuroprotective and antitumor effects. Molecules 2019, 24, 534. [Google Scholar] [CrossRef]
- Fang, X.-Y.; Song, R.; Chen, W.; Yang, Y.-Y.; Gu, Y.-H.; Shu, Y.-Q.; Wu, X.-D.; Wu, X.-F.; Sun, Y.; Shen, Y.; et al. PRL-3 Promotes the Malignant Progression of Melanoma via Triggering Dephosphorylation and Cytoplasmic Localization of NHERF1. J. Investig. Dermatol. 2015, 135, 2273–2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds rutin, quercetin, kaempferol, isorhamnetin and enzymes TpeBgl3, TthBgl3, AniBgl3, AteRha78, AniRha78, BthRha are available from the authors. |






| Aroma Component | Content (%) | |||
|---|---|---|---|---|
| Control | β-G | α-R | α-R & β-G | |
| 3-Methylbutyric acid | / | / | 0.51 ± 0.26 | 4.4 ± 1.65 |
| Trimethylsilylmethanol | 1.35 ± 0.99 | / | 0.44 ± 0.35 | / |
| 2-Methoxy-1,3-dioxolane | 2.02 ± 1.52 | / | 7.84 ± 1.99 | 8.54 ± 2.58 |
| Dihydroactinidiolide | 4.49 ± 1.46 | 0.795 ± 0.92 | 0.63 ± 0.28 | / |
| Vanillic acid | 1.66 ± 1.19 | 2.07 ± 1.33 | 1.16 ± 0.80 | 4.02 ± 1.14 |
| Vanillin | / | 0.61 ± 0.47 | 2.28 ± 0.89 | 1.45 ± 0.81 |
| Benzyl alcohol | / | 2.07 ± 1.55 | / | 4.94 ± 2.33 |
| p-Hydroxybenzyl alcohol | / | 6.08 ± 1.69 | / | 1.04 ± 1.00 |
| p-Hydroxybenzaldehyde | / | 4.89 ± 2.85 | 0.29 ± 0.24 | 8.78 ± 1.83 |
| Dihydro-β-ionone | / | 3.77 ± 1.89 | / | / |
| 3,4,5-Trimethoxybenzyl alcohol | / | 0.54 ± 0.54 | / | / |
| 5-Hydroxymethylfurfural | / | 0.59 ± 0.28 | 0.87 ± 0.27 | 0.72 ± 0.26 |
| Phenylacetic acid | / | / | 0.25 ± 0.12 | / |
| p-Hydroxyphenylethanol | / | 0.645 ± 0.31 | / | 0.8 ± 0.58 |
| Piperonylacetone | / | 0.42 ± 0.32 | 2.29 ± 1.18 | / |
| Total | 9.52 ± 2.24 | 22.47 ± 3.96 | 16.56 ± 4.36 | 34.69 ± 5.21 |
| Aglycone | Concentration (μg/mL) of Flavonoids in the Concentrated Tea Infusion | ||||||
|---|---|---|---|---|---|---|---|
| First Time | Second Time | Third Time | |||||
| Control | Enzyme | Control | Enzyme | Control | Enzyme | ||
| Brand I | Quercetin | 3.15 ± 1.95 | 149.68 ± 20.15 | 1.82 ± 0.76 | 142.49 ± 18.64 | 2.25 ± 1.43 | 49.88 ± 11.77 |
| Kaempferol | 1.54 ± 0.66 | 106.41 ± 13.52 | 2.10 ± 0.92 | 105.38 ± 12.01 | 3.38 ± 1.53 | 91.93 ± 10.14 | |
| Isorhamnetin | 1.17 ± 0.45 | 97.47 ± 11.04 | 1.31 ± 0.55 | 96.77 ± 11.24 | 1.27 ± 0.52 | 29.79 ± 4.86 | |
| Brand II | Quercetin | 3.05 ± 1.34 | 143.97 ± 16.15 | 1.94 ± 0.89 | 107.58 ± 11.56 | 1.89 ± 0.61 | 73.16 ± 8.37 |
| Kaempferol | 3.71 ± 1.73 | 167.12 ± 21.61 | 2.97 ± 1.30 | 134.02 ± 14.26 | 1.75 ± 0.59 | 142.70 ± 14.77 | |
| Isorhamnetin | 2.40 ± 0.98 | 167.14 ± 20.17 | 2.02 ± 0.91 | 125.62 ± 12.94 | 2.37 ± 0.87 | 103.34 ± 11.21 | |
| Brand III | Quercetin | 4.09 ± 2.23 | 238.10 ± 26.42 | 2.59 ± 1.16 | 22.33 ± 4.01 | 0.44 ± 0.16 | 8.53 ± 2.79 |
| Kaempferol | 3.78 ± 1.82 | 170.31 ± 17.52 | 2.93 ± 1.26 | 43.94 ± 5.24 | 3.95 ± 1.86 | 49.50 ± 6.11 | |
| Isorhamnetin | 1.73 ± 0.69 | 106.19 ± 11.38 | 1.09 ± 0.44 | 9.75 ± 2.85 | 0.79 ± 0.21 | 5.53 ± 2.35 | |
| Brand IV | Quercetin | 0.95 ± 0.26 | 157.06 ± 16.85 | 0.87 ± 0.32 | 53.55 ± 6.13 | 1.32 ± 0.53 | 8.73 ± 3.04 |
| Kaempferol | 3.10 ± 1.53 | 187.48 ± 21.33 | 1.16 ± 0.43 | 88.84 ± 8.24 | 2.89 ± 1.03 | 76.97 ± 7.21 | |
| Isorhamnetin | 1.81 ± 0.87 | 145.34 ± 15.04 | 0.31 ± 0.14 | 59.43 ± 6.44 | 1.42 ± 0.56 | 14.77 ± 3.56 | |
| TpeBgl3 [24] | TthBgl3 [23] | AniBgl3 [37] | AteRha78 [25] | AniRha78 [38] | BthRha [39] | |
|---|---|---|---|---|---|---|
| Type | GH3 | GH3 | GH3 | GH78 | GH78 | GH78 |
| Optimum temperature (°C) | 90 | 95 | 60 | 65 | 35 | 55 |
| Optimum pH | 5.0 | 5.0 | 4.0 | 6.5 | 6.5 | 6.5 |
| Km (mM) | 1.6 | 0.065 | 0.643 | 0.476 | 4.23 | 2.87 |
| Kcat (s−1) | / | 121 | / | 412 | / | 1743 |
| Vmax (μmol/min·mg) | 109 | / | 0.71 | / | 3.64 × 10−3 | / |
| Substrate specificity | PNPG 1 | PNPG | PNPG | PNPR 2 | PNPR | PNPR |
| Heat stability (h) 2 | 3 | 1 | 0.5 | 3 | 2 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Dong, Y.; Xie, Y.; Wang, L.; Wang, J.; Liu, Y.; Zhao, L.; Cao, F. Effects of β-glucosidase and α-rhamnosidase on the Contents of Flavonoids, Ginkgolides, and Aroma Components in Ginkgo Tea Drink. Molecules 2019, 24, 2009. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24102009
Fang X, Dong Y, Xie Y, Wang L, Wang J, Liu Y, Zhao L, Cao F. Effects of β-glucosidase and α-rhamnosidase on the Contents of Flavonoids, Ginkgolides, and Aroma Components in Ginkgo Tea Drink. Molecules. 2019; 24(10):2009. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24102009
Chicago/Turabian StyleFang, Xianying, Yurong Dong, Yingying Xie, Lei Wang, Jingqiu Wang, Yuechen Liu, Linguo Zhao, and Fuliang Cao. 2019. "Effects of β-glucosidase and α-rhamnosidase on the Contents of Flavonoids, Ginkgolides, and Aroma Components in Ginkgo Tea Drink" Molecules 24, no. 10: 2009. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24102009