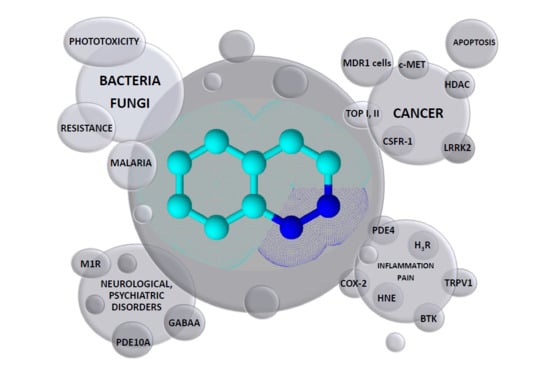
Cinnoline Scaffold—A Molecular Heart of Medicinal Chemistry?
Abstract
:1. Introduction
2. Biological Activity of Cinnoline Derivatives
2.1. Antimicrobial Activity
2.2. Analgesic and Antiinflamatory Activities
2.3. Potential for Neurological Disorders
2.4. Anticancer Properties
2.5. Miscellaneous
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lewgowd, W.; Stanczak, A. Cinnoline derivatives with biological activity. Arch. Pharm. 2007, 340, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Castle, N.R. The Chemistry of Heterocyclic Compounds; John Wiley & Sons: New York, NY, USA, 1973; Volume 27, Chapter 1; pp. 1–231. [Google Scholar]
- Vinogradova, O.V.; Balova, I.A. Methods for the synthesis of cinnolines. Chem. Heterocycl. Compd. 2008, 44, 501–522. [Google Scholar] [CrossRef]
- Kumari, S.; Kishore, D.; Paliwal, S.; Chauhan, R.; Dwivedi, J.; Mishra, A. Transition metal-free one-pot synthesis of nitrogen-containing heterocycles. Mol. Diver. 2016, 20, 185–232. [Google Scholar] [CrossRef] [PubMed]
- Muralirajan, K.; Cheng, C.H. Rhodium(III)-catalyzed synthesis of cinnolinium salts from azobenzenes and alkynes: Application to the synthesis of indoles and cinnolines. Chem. Eur. J. 2013, 19, 6198–6202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wu, Q.; Huang, X.; Song, F.; Lv, T.; You, J. A general method to diverse cinnolines and cinnolinium salts. Chem. Eur. J. 2013, 19, 6239–6244. [Google Scholar] [CrossRef] [PubMed]
- Kiriazis, A.; Rüffer, T.; Jäntti, S.; Lang, H.; Yli-Kauhaluoma, J. Stereoselective aza Diels-Alder reaction on solid phase: A facile synthesis of hexahydrocinnoline derivatives. J. Comb. Chem. 2007, 9, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Mathew, T.; Papp, A.Á.; Paknia, F.; Fustero, S.; Surya Prakash, G.K. Benzodiazines: Recent synthetic advances. Chem. Soc. Rev. 2017, 46, 3060–3094. [Google Scholar] [CrossRef]
- Haddadin, M.J.; Zerdan, R.M.B.; Kurth, M.J.; Fettinger, J.C. Efficient syntheses of the unknown quinolino [2,3-c] cinnolines; Synthesis of neocryptolepines. Org. Lett. 2010, 12, 5502–5505. [Google Scholar] [CrossRef]
- Chen, C.J.; Deng, A.J.; Liu, C.; Shi, R.; Qin, H.L.; Wang, A.P. Hepatoprotective activity of cichorium endivia L. extract and its chemical constituents. Molecules 2011, 16, 9049–9066. [Google Scholar] [CrossRef]
- Satyanarayana, M.; Feng, W.; Cheng, L.; Liu, A.A.; Tsai, Y.-C.; Liu, L.F.; LaVoie, E.J. Syntheses and biological evaluation of topoisomerase I-targeting agents related to 11-[2-(N, N-dimethylamino) ethyl]-2,3-dimethoxy-8,9-methylenedioxy-11H-isoquino [4,3-c] cinnolin-12-one (ARC-31). Bioorg. Med. Chem. 2008, 16, 7824–7831. [Google Scholar] [CrossRef]
- Devine, W.; Woodring, J.L.; Swaminathan, U.; Amata, E.; Patel, G.; Erath, J.; Roncal, N.E.; Lee, P.J.; Leed, S.E.; Rodriguez, A.; et al. Protozoan parasite growth inhibitors discovered by cross-screening yield potent scaffolds for lead discovery. J. Med. Chem. 2015, 58, 5522–5537. [Google Scholar] [CrossRef] [PubMed]
- Alhambra, C.; Becker, C.; Blake, T.; Chang, A.; Damewood, J.R.; Daniels, T.; Dembofsky, B.T.; Gurley, D.A.; Hall, J.E.; Herzog, K.J.; et al. Development and SAR of functionally selective allosteric modulators of GABAA receptors. Bioorg. Med. Chem. 2011, 19, 2927–2938. [Google Scholar] [CrossRef] [PubMed]
- Barlaam, B.; Cadogan, E.; Campbell, A.; Colclough, N.; Dishington, A.; Durant, S.; Goldberg, K.; Hassall, L.A.; Hughes, G.D.; MacFaul, P.A.; et al. Discovery of a series of 3-cinnoline carboxamides as orally bioavailable, highly potent, and selective ATM inhibitors. ACS Med. Chem. Lett. 2018, 9, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Szumilak, M.; Szulawska-Mroczek, A.; Koprowska, K.; Stasiak, M.; Lewgowd, W.; Stanczak, A.; Czyz, M. Synthesis and in vitro biological evaluation of new polyamine conjugates as potential anticancer drugs. Eur. J. Med. Chem. 2010, 45, 5744–5751. [Google Scholar] [CrossRef] [PubMed]
- Szumilak, M.; Lewgowd, W.; Stanczak, A. In silico ADME studies of polyamine conjugates as potential anticancer drugs. Acta Pol. Pharm. 2016, 73, 1190–1199. [Google Scholar]
- Wiederhold, N.P. Antifungal resistance: Current trends and future strategies to combat. Infect Drug Res. 2017, 10, 249–259. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Cinobac. Available online: https://www.rxlist.com/cinobac-drug.htm#clinpharm (accessed on 31 March 2019).
- Vargas, F.; Zoltan, T.; Rivas, C.; Ramirez, A.; Cordero, T.; Díaz, Y.; Izzo, C.; Cárdenas, Y.M.; López, V.; Gómez, L.; et al. Synthesis, primary photophysical and antibacterial properties of naphthyl ester cinoxacin and nalidixic acid derivatives. J. Photochem. Photobiol. B 2008, 92, 83–90. [Google Scholar] [CrossRef]
- Ryu, C.-K.; Lee, J.Y. Synthesis and antifungal activity of 6-hydroxycinnolines. Bioorg. Med. Chem. Lett. 2006, 16, 1850–1853. [Google Scholar] [CrossRef]
- Vikas, S.; Darbhamulla, S. Synthesis, characterization and biological activities of substituted cinnoline sulphonamides. Afr. Health Sci. 2009, 9, 275–278. [Google Scholar]
- Gautam, N.; Chourasia, O.P. Synthesis, antimicrobial and insecticidal activity of some new cinnoline based chalcones and cinnolinc based pyrazoline derivatives. Indian J. Chem. B 2010, 49, 830–835. [Google Scholar]
- Unnissa, S.H.; Ravi, T.K. Synthesis and screening of pyrazole based cinnoline derivatives for its anti-tubercular and anti-fungal activity. J. Chem. Pharm. Res. 2015, 7, 957–963. [Google Scholar]
- Unnissa, S.H.; Nisha, N.; Reddy, G.K. Synthesis and in vitro antimicrobial evaluation including anti-malarial activity of pyrazole based novel cinnoline derivatives. J. Appl. Pharm. Sci. 2015, 15, 121–126. [Google Scholar] [CrossRef]
- Parasuraman, P.; Shanmugarajan, R.S.; Aravazhi, T.; Nehru, K.; Mathiazhaga, T.; Rajakumari, R. Synthesis, characterization and antimicrobial evaluation of some substituted 4-aminocinnoline-3- carboxamide derivatives. Int. J. Pharm. Life Sci. 2012, 3, 1430–1436. [Google Scholar]
- Varshney, S.; Saxena, V. Design, synthesis, characterization and biological evaluation of some novel cinnolo piperazine derivatives. Int. J. Pharm. Pharm. Sci. 2014, 6, 245–248. [Google Scholar]
- Mishra, P.; Middha, A.; Saxena, V.; Saxena, A. Synthesis, biological evaluation and comparative study of some cinnoline derivatives. UK J. Pharm. Biosci. 2016, 4, 74–80. [Google Scholar] [CrossRef]
- Dawadi, S.; Boshoff, H.I.M.; Park, S.W.; Schnappinger, D.; Aldrich, C.C. Conformationally constrained cinnolinone nucleoside analogues as siderophore biosynthesis inhibitors for tuberculosis. ACS Med. Chem. Lett. 2018, 9, 386–391. [Google Scholar] [CrossRef]
- Glinka, T.; Lomovskaya, O.; Bostian, K.; Wallace, D.M. Preparation of Peptide Polybasic Bacterial Efflux Pump Inhibitors for Enhancing Levofloxacin Potency in Treating Bacterial Infections. WO Patent 2008141010A2, 20 November 2008. [Google Scholar]
- Chaudhary, J.; Patel, K.; Patel, C.N. Synthesis and biological screening of some cinnoline derivatives. Int. J. Univers. Pharm. Biol. Sci. 2014, 3, 128–140. [Google Scholar]
- Tonk, R.K.; Bawa, S.; Chawla, G.; Deora, G.S.; Kumar, S.; Rathore, V.; Mulakayala, N.; Rajaram, A.; Kalle, A.M.; Afzal, O. Synthesis and pharmacological evaluation of pyrazolo[4,3-c]cinnoline derivatives as potential anti-inflammatory and antibacterial agents. Eur. J. Med. Chem. 2012, 57, 176–184. [Google Scholar] [CrossRef]
- Lunniss, C.; Eldred, C.; Aston, N.; Craven, A.; Gohil, K.; Judkins, B.; Keeling, S.; Ranshaw, L.; Robinson, E.; Shipley, T.; et al. Addressing species specific metabolism and solubility issues in a quinoline series of oral PDE4 inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 137–140. [Google Scholar] [CrossRef]
- Houslay, M.D.; Schafer, P.; Zhang, K.Y.J. Keynote review: Phosphodiesterase-4 as a therapeutic target. Drug Discov. Today 2005, 10, 1503–1519. [Google Scholar] [CrossRef]
- Gomtsyan, A.; Bayburt, E.K.; Schmidt, R.G.; Zheng, G.Z.; Perner, R.J.; Didomenico, S.; Koenig, J.R.; Turner, S.; Jinkerson, T.; Drizin, I.; et al. Novel transient receptor potential vanilloid 1 receptor antagonists for the treatment of pain: structure−activity relationships for ureas with quinoline, isoquinoline, quinazoline, phthalazine, quinoxaline, and cinnoline moieties. J. Med. Chem. 2005, 48, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, J.A.; Huang, T.; Balazs, M.; Barbosa, J.; Barck, K.H.; Bravo, B.J.; Carano, R.A.D.; Darrow, J.; Davies, D.R.; DeForge, L.E.; et al. Specific Btk inhibition suppresses B cell- and myeloid cell-mediated arthritis. Nat. Chem. Biol. 2011, 7, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.D.; Sabat, M.; Smith, C.; Wang, H.; Chen, Y.K.; Kanouni, T. Preparation of Cinnolinecarboxamides as BKT Inhibitors. WO Patent 2013148603A1, 03 October 2013. [Google Scholar]
- Smith, C.R.; Dougan, D.R.; Komandla, M.; Kanouni, T.; Knight, B.; Lawson, J.D.; Sabat, M.; Taylor, E.R.; Vu, P.; Wyrick, C. Fragment-based discovery of a small molecule inhibitor of Bruton’s tyrosine kinase. J. Med. Chem. 2015, 58, 5437–5444. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, L.; Giovannoni, M.P.; Schepetkin, I.A.; Quinn, M.T.; Khlebnikov, A.I.; Cilibrizzi, A.; Dal Piaz, V.; Graziano, A.; Vergelli, C. Design, synthesis and evaluation of N-benzoylindazole derivatives and analogues as inhibitors of human neutrophil elastase. Bioorg. Med. Chem. 2011, 19, 4460–4472. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, L.; Schepetkin, I.A.; Cilibrizzi, A.; Graziano, A.; Vergelli, C.; Gionni, D.; Khlebnikov, A.I.; Quinn, M.T.; Giovannoni, M.P. Optimization of N-Benzoylindazole derivatives as inhibitors of human neutrophil elastase. J. Med. Chem. 2013, 56, 6259–6272. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, M.P.; Schepetkin, I.A.; Crocetti, L.; Ciciani, G.; Cilibrizzi, A.; Guerrini, G.; Khlebnikov, A.I.; Quinn, M.T.; Vergelli, C. Cinnoline derivatives as human neutrophil elastase inhibitors. J. Enzyme Inhib. Med. Chem. 2016, 31, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, G.; Srinivas, B.; Sastry, K.V.; Vijaya, K. Synthesis of novel cinnoline fused mannich bases: Pharmacological evaluation of antibacterial, analgesic and anti-inflammatory activity. IJPCR 2017, 9, 515–520. [Google Scholar] [CrossRef]
- Woll, M.G.; Amedzo, L.; Babu, S.; Barraza, S.J.; Bhattacharyya, A.; Karp, G.M.; Mazzotti, A.R.; Narasimhan, J.; Patel, J.; Turpoff, A.; et al. Preparation of Heteroaryl Compounds for Treating Huntington’s Disease. WO Patent 2018226622A1, 13 December 2018. [Google Scholar]
- Beshore, D.C.; Kuduk, S.D. Pyrazolo [4,3-c] Cinnolin-3-One Derivatives as M1 Receptor Positive Allosteric Modulators and Their Preparation, Pharmaceutical Compositions and Use in the Treatment of Diseases. WO Patent 2010096338A1, 26 August 2010. [Google Scholar]
- Amer, A.M.; El-Farargy, A.F.; Yousif, N.M.; Fayed, A.A. Synthesis and pharmacological activities of some dibenzopyrazolocinnolines and dibenzopyridazoquinoxalines. Chem. Heterocycl. Compd. 2011, 47, 101–107. [Google Scholar] [CrossRef]
- Hatcher, J.M.; Choi, H.G.; Alessi, D.R.; Gray, N.S. Small-molecule inhibitors of LRRK2. Adv. Neurobiol. 2017, 14, 241–264. [Google Scholar] [CrossRef]
- Aubele, D.L.; Usgarofalo, A.W.; Bowers, S.; Ustruong, A.P.; Usye, X.M.; Franzini, M.; Adler, M.; Usnietz, R.J.; Usprobst, G. Preparation of Cinnoline Derivatives as Inhibitors of LRRK2 Kinase Activity. WO Patent 2012162254A1, 29 November 2012. [Google Scholar]
- Garofalo, A.W.; Adler, M.; Aubele, D.L.; Bowers, S.; Franzini, M.; Goldbach, E.; Lorentzen, C.; Neitz, R.J.; Probst, G.D.; Quinn, K.P.; et al. Novel cinnoline-based inhibitors of LRRK2 kinase activity. Bioorg. Med. Chem. Lett. 2013, 23, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Zagórska, A.; Partyka, A.; Bucki, A.; Gawalska, A.; Czopek, A.; Pawłowski, M. Phosphodiesterase 10 inhibitors–novel perspectives for psychiatric and neurodegenerative drug discovery. Curr. Med. Chem. 2018, 25, 3455–3481. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Kunz, R.K.; Rumfelt, S.; Chen, N.; Burli, R.; Li, C.; Andrews, K.L.; Zhang, J.D.; Chmait, S.; Kogan, J.; et al. Discovery of potent, selective, and metabolically stable 4-(pyridin-3-yl) cinnolines as novel phosphodiesterase 10A (PDE10A) inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 2262–2265. [Google Scholar] [CrossRef]
- Yang, H.; Murigi, F.N.; Wang, Z.J.; Li, J.F.; Jin, H.J.; Tu, Z.D. Synthesis and in vitro characterization of cinnoline and benzimidazole analogues as phosphodiesterase 10A inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Kunz, R.K.; Rumfelt, S.; Andrews, K.L.; Li, C.; Hitchcock, S.A.; Lindstrom, M.; Treanor, J. Use of structure based design to increase selectivity of pyridyl-cinnoline phosphodiesterase 10A (PDE10A) inhibitors against phosphodiesterase 3 (PDE3). Bioorg. Med. Chem. Lett. 2012, 22, 6938–6942. [Google Scholar] [CrossRef] [PubMed]
- Geneste, H.; Drescher, K.; Jakob, C.; Laplanche, L.; Ochse, M.; Torrent, M. Novel, potent, selective, and brain penetrant phosphodiesterase 10A inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Geneste, H.; Ochse, M.; Drescher, K.; Jakob, C. Preparation of 3H-Pyrazolo [3,4-c] Cinnoline Derivatives as Phosphodiesterase Type 10A Inhibitors. WO Patent 2014027078A1, 20 February 2014. [Google Scholar]
- Josef, K.A.; Aimone, L.D.; Lyons, J.; Raddatz, R.; Hudkins, R.L. Synthesis of constrained benzocinnolinone analogues of CEP-26401 (irdabisant) as potent, selective histamine H3 receptor inverse agonists. Bioorg. Med. Chem. Lett. 2012, 22, 4198–4202. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.R.; Tallapragada, V.J.; Chebib, M.; Johnston, G.A.R.; Hanrahan, J.R. GABA allosteric modulators: An overview of recent developments in non-benzodiazepine modulators. Eur. J. Med. Chem. 2019, 171, 434–461. [Google Scholar] [CrossRef] [PubMed]
- Chapdelaine, M.J.; Ohnmacht, C.J.; Becker, C.; Chang, H.-F.; Dembofsky, B.T. Preparation of Novel Cinnoline Compounds for Treating Anxiety, Depression and Cognition Disorders. U.S. Patent 20070142328A1, 16 September 2007. [Google Scholar]
- Jucaite, A.; Cselenyi, Z.; Lappalainen, J.; McCarthy, D.J.; Lee, C.M.; Nyberg, S.; Varnas, K.; Stenkrona, P.; Halldin, C.; Cross, A.; et al. GABA(A) receptor occupancy by subtype selective GABA(A α 2,3) modulators: PET studies in humans. Psychopharmacology 2017, 234, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jacobs, G.; de Kam, M.; Jaeger, J.; Lappalainen, J.; Maruff, P.; Smith, M.A.; Cross, A.J.; Cohen, A.; van Gerven, J. The central nervous system effects of the partial GABA-A α (2,3)-selective receptor modulator AZD7325 in comparison with lorazepam in healthy males. Br. J. Clin. Pharmacol. 2014, 78, 1298–1314. [Google Scholar] [CrossRef]
- Te Beek, E.T.; Chen, X.; Jacobs, G.E.; Nahon, K.J.; de Kam, M.L.; Lappalainen, J.; Cross, A.J.; van Gerven, J.M.A.; Hay, J.L. The effects of the nonselective benzodiazepine lorazepam and the α (2)/α (3) subunit-selective GABA(A) receptor modulators AZD7325 and AZD6280 on plasma prolactin levels. Clin. Pharmacol. Ther. 2015, 4, 149–154. [Google Scholar] [CrossRef]
- Artelsmair, M.; Gu, C.; Lewis, R.J.; Elmore, C.S. Synthesis of C-14 labeled GABAA α2/α3 selective partial agonists and the investigation of late-occurring and long-circulating metabolites of GABAA receptor modulator AZD7325. J. Label. Compd. Radiopharm. 2018, 61, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Artelsmair, M.; Elmore, C.S.; Lewis, R.J.; Davis, P.; Hall, J.E.; Dembofsky, B.T.; Christoph, G.; Smith, M.A.; Chapdelaine, M.; et al. Late-occurring and long-circulating metabolites of GABAAα2,3 receptor modulator AZD7325 involving metabolic cyclization and aromatization: Relevance to MIST analysis and application for patient compliance. Drug Metab. Dispos. 2018, 46, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, M.; Barcelo, M.; Carro, L.; Masaguer, C.F.; Ravina, E. Synthesis and biological evaluation of new quinazoline and cinnoline derivatives as potential atypical antipsychotics. Chem. Biodivers. 2006, 3, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Singh, S.K.; Liu, A.; Li, T.-K.; Liu, L.F.; LaVoie, E.J. Substituted dibenzo [c,h] cinnolines: Topoisomerase I-targeting anticancer agents. Bioorg. Med. Chem. 2003, 11, 1475–1491. [Google Scholar] [CrossRef]
- Ruchelman, A.L.; Singh, S.K.; Wu, X.; Ray, A.; Yang, J.-M.; Li, T.-K.; Liu, A.; Liu, L.F.; LaVoie, E.J. Diaza-and triazachrysenes: Potent topoisomerase-targeting agents with exceptional antitumor activity against the human tumor xenograft, MDA-MB-435. Bioorg. Med. Chem. Lett. 2002, 12, 3333–3336. [Google Scholar] [CrossRef]
- Ruchelman, A.L.; Singh, S.K.; Ray, A.; Wu, X.; Yang, J.-M.; Zhou, N.; Liu, A.; Liu, L.F.; LaVoie, E.J. 11H-Isoquino [4,3-c] cinnolin-12-ones: Novel anticancer agents with potent topoisomerase I-targeting activity and cytotoxicity. Bioorg. Med. Chem. 2004, 12, 795–806. [Google Scholar] [CrossRef]
- Feng, W.; Satyanarayana, M.; Tsai, Y.-C.; Liu, A.A.; Liu, L.F.; LaVoie, E.J. Facile formation of hydrophilic derivatives of 5H-8,9-dimethoxy-5- [2-(N,N-dimethylamino)ethyl]-2,3-methylenedioxydibenzo [c,h] [1,6]naphthyridin-6-one (ARC-111) and its 12-aza analog via quaternary ammonium intermediates. Bioorg. Med. Chem. Lett. 2008, 18, 3570–3572. [Google Scholar] [CrossRef]
- Zoidis, G.; Sosic, A.; Da Ros, S.; Gatto, B.; Sissi, C.; Palluotto, F.; Carotti, A.; Catto, M. Indenocinnoline derivatives as G-quadruplex binders, topoisomerase Hoc inhibitors and antiproliferative agents. Bioorg. Med. Chem. 2017, 25, 2625–2634. [Google Scholar] [CrossRef]
- Borowski, E.; Stefanska, B.; Dzieduszycka, M.; Cybulski, M.; Szelejewski, W.; Obukowicz, J.; Bontemps-Gracz, M. Preparation of Asymmetrically Substituted Anthrapyridazone Derivatives as Cytostatics. WO Patent 2012141604A1, 18 October 2012. [Google Scholar]
- Borowski, E.; Stefanska, B.; Dzieduszycka, M.; Cybulski, M.; Szelejewski, W.; Obukowicz, J.; Bontemps-Gracz, M.; Wysocka, M.; Mazerski, J.; Punda, P.; et al. Asymmetrically Substituted Anthrapyridazone Derivatives as Cytostatics. U.S. Patent 9096536B2, 08 April 2015. [Google Scholar]
- Stefańska, B.; Arciemiuk, M.; Bontemps-Gracz, M.M.; Dzieduszycka, M.; Kupiec, A.; Martelli, S.; Borowski, E. Synthesis and biological evaluation of 2,7-Dihydro-3H-dibenzo [de,h] cinnoline-3,7-dione derivatives, a novel group of anticancer agents active on a multidrug resistant cell line. Bioorg. Med. Chem. 2003, 11, 561–572. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, A.; Muscarella, M.; Spano, V.; Montalbano, A.; Barraja, P.; Salvador, A.; Vedaldi, D.; Cirrincione, G.; Diana, P. 11H-Pyrido [3′,2′:4,5] pyrrolo [3,2-c] cinnoline and pyrido [3′,2′:4,5] pyrrolo [1,2-c][1,2,3] benzotriazine: Two new ring systems with antitumor activity. J. Med. Chem. 2014, 57, 9495–9511. [Google Scholar] [CrossRef] [PubMed]
- Pike, K.G.; Barlaam, B.C. Preparation of Cinnolin-4-Amine Compounds as ATM Kinase Inhibitors and Their Use in Treating Cancer. WO Patent 2017162605A1, 28 September 2017. [Google Scholar]
- Pike, K.G.; Barlaam, B.C. Preparation of 1,3-Dihydroimidazo [4,5-c] Cinnolin-2-Ones for the Treatment and Prevention of Cancer. WO Patent 2019057757A1, 28 March 2019. [Google Scholar]
- Hume, D.A.; MacDonald, K.P.A. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 2012, 119, 1810. [Google Scholar] [CrossRef] [PubMed]
- Daly, K.; Del Valle, D.; Scott, D.; Ye, Q. Preparation of Cinnolinecarboxamides as Colony Stimulating Factor 1 Receptor (CSF-1R) Kinase Inhibitors. WO Patent 2009136191A1, 12 November 2009. [Google Scholar]
- Scott, D.A.; Dakin, L.A.; Del Valle, D.J.; Bruce Diebold, R.; Drew, L.; Gero, T.W.; Ogoe, C.A.; Omer, C.A.; Repik, G.; Thakur, K.; et al. 3-Amido-4-anilinocinnolines as a novel class of CSF-1R inhibitor. Bioorg. Med. Chem. Lett. 2011, 21, 1382–1384. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Dakin, L.A.; Daly, K.; Del Valle, D.J.; Diebold, R.B.; Drew, L.; Ezhuthachan, J.; Gero, T.W.; Ogoe, C.A.; Omer, C.A.; et al. Mitigation of cardiovascular toxicity in a series of CSF-1R inhibitors, and the identification of AZD7507. Bioorg. Med. Chem. Lett. 2013, 23, 4591–4596. [Google Scholar] [CrossRef] [PubMed]
- Parizadeh, S.M.; Jafarzadeh-Esfehani, R.; Fazilat-Panah, D.; Hassanian, S.M.; Shahidsales, S.; Khazaei, M.; Parizadeh, S.M.R.; Ghayour-Mobarhan, M.; Ferns, G.A.; Avan, A. The potential therapeutic and prognostic impacts of the c-MET/HGF signaling pathway in colorectal cancer. IUBMB Life 2019. [Google Scholar] [CrossRef]
- Gong, P.; Zhao, Y.; Liu, Y.; Zhai, X. Quinoline and cinnoline compounds as c-Met kinase inhibitors and their preparation, pharmaceutical compositions and use in the treatment of hyperplastic diseases. CN Patent 102643268A, 21 May 2012. [Google Scholar]
- Li, S.; Zhao, Y.F.; Wang, K.W.; Gao, Y.L.; Han, J.M.; Cui, B.B.; Gong, P. Discovery of novel 4-(2-fluorophenoxy) quinoline derivatives bearing 4-oxo-1,4-dihydrocinnoline-3-carboxamide moiety as c-Met kinase inhibitors. Bioorg. Med. Chem. 2013, 21, 2843–2855. [Google Scholar] [CrossRef] [PubMed]
- Mohareb, R.M.; Al Farouk, F.O.; Wardakhan, W.W. Uses of dimedone for the synthesis of new heterocyclic derivatives with anti-tumor, c-Met, tyrosine, and Pim-1 kinases inhibitions. Med. Chem. Res. 2018, 27, 1984–2003. [Google Scholar] [CrossRef]
- Dang Thi, T.A.; Decuyper, L.; Thi Phuong, H.; Vu Ngoc, D.; Thanh Nguyen, H.; Thanh Nguyen, T.; Do Huy, T.; Huy Nguyen, H.; D’Hooghe, M.; Van Nguyen, T. Synthesis and cytotoxic evaluation of novel dihydrobenzo [h] cinnoline-5,6-diones. Tetrahedron Lett. 2015, 56, 5855–5858. [Google Scholar] [CrossRef]
- Al-zagameem, A.S.; El-Abadelah, M.M.; Zihlif, M.A.; Naffa, R.G.; Al-Smadi, M.L.; Mubarak, M.S. Synthesis and bioassay of novel substituted pyrano [2,3-f] cinnoline-2-ones. J. Heterocycl. Chem. 2016, 53, 1771–1777. [Google Scholar] [CrossRef]
- Awad, E.D.; El-Abadelah, M.M.; Matar, S.; Zihlif, M.A.; Naffa, R.G.; Al-Momani, E.Q.; Mubarak, M.S. Synthesis and biological activity of some 3-(4-(Substituted)-piperazin-1-yl) cinnolines. Molecules 2012, 17, 227–239. [Google Scholar] [CrossRef]
- Mohareb, R.; Moustafa, H. Use of 2-aminoprop-1-ene-1,1,3-tricarbonitrile for the synthesis of tetrahydronaphthalene, hexahydroisoquinoline and hexahydrocinnoline derivatives with potential antitumor activities. Acta Pharm. 2011, 61, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unnissa, S.H.; Rose, S. Drug design, development and screening of pyrazolo pyridazine as potential agent for treatment of breast cancer. J. Chem. Pharm. Res. 2015, 7, 966–971. [Google Scholar]
- Al-Qtaitat, M.A.; El-Abadelah, M.M.; Sabri, S.S.; Matar, S.A.; Hammad, H.M.; Mubarak, M.S. Synthesis, characterization and bioactivity of novel bicinnolines having 1-piperazinyl moieties. J. Heterocycl. Chem. 2019, 56, 158–164. [Google Scholar] [CrossRef]
- Stein, P.; Daines, R.; Sprous, D.; O’Grady, H. Preparation of Cyclic Hydrazone Compounds and Their Use in Inhibiting Taste and Treating Diabetes and Other Disorders. WO Patent 2010132615A1, 18 November 2010. [Google Scholar]
- Garcia Collazo, A.M.; Koch, E.K.; Lofstedt, A.J.; Cheng, A.; Hansson, T.F.; Zamaratski, E. Preparation of Quinolines, Indazoles, and Their Analogs as Thyroid Receptor Agonists. WO Patent 2007003419A1, 11 January 2007. [Google Scholar]
- Betschart, C.; Cotesta, S.; Hintermann, S.; Wagner, J.; Roy, B.L.; Gerspacher, M.; Von Matt, A. Preparation of 4-Aryl-Butane-1,3-Diamides as Orexin Receptors Antagonists. WO Patent 2011073316A1, 23 June 2011. [Google Scholar]
- Aissaoui, H.; Boss, C.; Gude, M.; Koberstein, R.; Sifferlen, T. Preparation of Azetidine Compounds as Orexin Receptor Antagonists. WO Patent 2008020405A2, 21 February 2008. [Google Scholar]
- Aissaoui, H.; Boss, C.; Brotschi, C.; Koberstein, R.; Siegrist, R.; Sifferlen, T.; Trachsel, D.; Williams, J.T. Preparation of Phenethylamide Derivatives and Their Heterocyclic Analogs as Orexin Receptor Antagonists. WO Patent 2010044054A1, 22 April 2010. [Google Scholar]
- Chai, W.; Letavic, M.A.; Ly, K.S.; Pippel, D.J.; Rudolph, D.A.; Sappey, K.C.; Savall, B.M.; Shah, C.R.; Shireman, B.T.; Soyode-Johnson, A.; et al. Preparation of Disubstituted Octahydropyrrolo [3,4-c] Pyrroles as Orexin Receptor Modulators. WO Patent 2011050198A1, 28 April 2011. [Google Scholar]
- Letavic, M.; Rudolph, D.A.; Savall, B.M.; Shireman, B.T.; Swanson, D. Disubstituted Octahydropyrrolo [3,4-c] Pyrroles as Orexin Receptor Modulators and Their Preparation. WO Patent 2012145581A1, 26 October 2012. [Google Scholar]
- Rusche, J.R.; Peet, N.P.; Hopper, A.T. Preparation of 6-Aminohexanoic Acid Derivatives as HDAC Inhibitors. WO Patent 2010028192A1, 11 March 2010. [Google Scholar]
- Hu, B.; Unwalla, R.; Collini, M.; Quinet, E.; Feingold, I.; Goos-Nilsson, A.; Wihelmsson, A.; Nambi, P.; Wrobel, J. Discovery and SAR of cinnolines/quinolines as liver X receptor (LXR) agonists with binding selectivity for LXRβ. Bioorg. Med. Chem. 2009, 17, 3519–3527. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Han, S.; Kim, S.H.; Wang, S.; Zhu, Y. Preparation of Nitrogen Containing Bicyclic Compounds as Somatostatin Modulators, Especially SSTR2 Agonists, and Their Therapeutic Use. WO Patent 2018013676A1, 18 January 2018. [Google Scholar]
- Abouzid, K.; Shouman, S. Design, synthesis and in vitro antitumor activity of 4-aminoquinoline and 4-aminoquinazoline derivatives targeting EGFR tyrosine kinase. Bioorg. Med. Chem. 2008, 16, 7543–7551. [Google Scholar] [CrossRef] [PubMed]








































© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szumilak, M.; Stanczak, A. Cinnoline Scaffold—A Molecular Heart of Medicinal Chemistry? Molecules 2019, 24, 2271. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24122271
Szumilak M, Stanczak A. Cinnoline Scaffold—A Molecular Heart of Medicinal Chemistry? Molecules. 2019; 24(12):2271. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24122271
Chicago/Turabian StyleSzumilak, Marta, and Andrzej Stanczak. 2019. "Cinnoline Scaffold—A Molecular Heart of Medicinal Chemistry?" Molecules 24, no. 12: 2271. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24122271