Diallyl Disulfide Induces Apoptosis and Autophagy in Human Osteosarcoma MG-63 Cells through the PI3K/Akt/mTOR Pathway
Abstract
:1. Introduction
2. Results
2.1. DADs Inhibit Osteosarcoma Cell Viability and Induces Cell Cycle Arrest at the G2/M Phase
2.2. DADs Induce Apoptosis of Osteosarcoma Cells
2.3. DADs Induce Autophagy of Osteosarcoma Cells
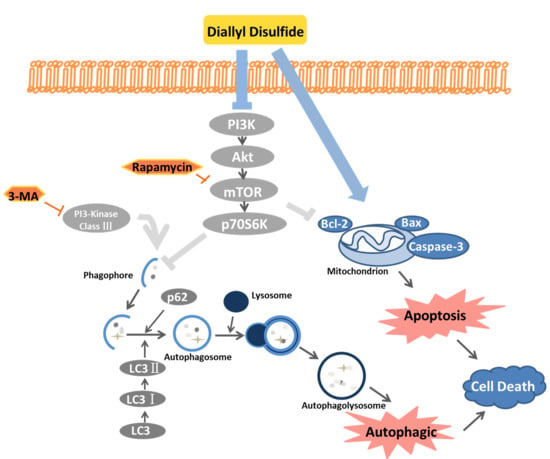
2.4. DADs Induces Apoptosis and Autophagy by Inhibiting the PI3K/Akt/mTOR Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Clone Formation Assay
4.5. Cell Cycle Analysis
4.6. Apoptosis Flow-Cytometry Assay
4.7. Western Blot Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raymond, A.K.; Jaffe, N. Osteosarcoma multidisciplinary approach to the management from the pathologist’s perspective.%a raymond ak. Cancer Treat. Res. 2009, 152, 63–84. [Google Scholar] [PubMed]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Durfee, R.A.; Mohammed, M.; Luu, H.H. Review of osteosarcoma and current management. Rheumatol. Ther. 2016, 3, 221–243. [Google Scholar] [CrossRef] [PubMed]
- Link, M.P.; Goorin, A.M.; Miser, A.W.; Green, A.A.; Pratt, C.B.; Belasco, J.B.; Pritchard, J.; Malpas, J.S.; Baker, A.R.; Kirkpatrick, J.A.; et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N. Engl. J. Med. 1986, 314, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Y, L.; Z, W.; J, L.; X, S. Diallyl disulfide suppresses foxm1-mediated proliferation and invasion in osteosarcoma by upregulating mir-134. J. Cell. Biochem. 2018, undefined, undefined. [Google Scholar]
- Ji, X.X.; Liu, F.; Xia, H.; He, J.; Tan, H.; Yi, L.; Su, Q. [downregulation of mcl-1 by diallyl disulfide induces g2/m arrest in human leukemia k562 cells and its mechanism]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2018, 26, 750–755. [Google Scholar] [PubMed]
- Wu, X.J.; Kassie, F.; Mersch-Sundermann, V. The role of reactive oxygen species (ros) production on diallyl disulfide (dads) induced apoptosis and cell cycle arrest in human a549 lung carcinoma cells. Mutat. Res. 2005, 579, 115–124. [Google Scholar] [CrossRef]
- Yang, J.S.; Chen, G.W.; Hsia, T.C.; Ho, H.C.; Ho, C.C.; Lin, M.W.; Lin, S.S.; Yeh, R.D.; Ip, S.W.; Lu, H.F.; et al. Diallyl disulfide induces apoptosis in human colon cancer cell line (colo 205) through the induction of reactive oxygen species, endoplasmic reticulum stress, caspases casade and mitochondrial-dependent pathways. Food Chem. Toxicol. 2009, 47, 171–179. [Google Scholar] [CrossRef]
- Lu, H.F.; Sue, C.C.; Yu, C.S.; Chen, S.C.; Chen, G.W.; Chung, J.G. Diallyl disulfide (dads) induced apoptosis undergo caspase-3 activity in human bladder cancer t24 cells. Food Chem. Toxicol. 2004, 42, 1543–1552. [Google Scholar] [CrossRef]
- Lin, Y.T.; Yang, J.S.; Lin, S.Y.; Tan, T.W.; Ho, C.C.; Hsia, T.C.; Chiu, T.H.; Yu, C.S.; Lu, H.F.; Weng, Y.S.; et al. Diallyl disulfide (dads) induces apoptosis in human cervical cancer ca ski cells via reactive oxygen species and ca2+-dependent mitochondria-dependent pathway. Anticancer Res. 2008, 28, 2791–2799. [Google Scholar]
- Sherr, C.J. Cancer cell cycles. Science 1996, 274, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, L.H.; Kastan, M.B. Cell-cycle control and cancer. Science 1994, 266, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.R.; Zhang, R.; Feng, C.; Zhang, J.; Liu, D.; Xu, K.; Wang, X.; Zhang, S.Q.; Li, Z.F.; Liu, X.L.; et al. Diallyl disulfide induces g2/m arrest and promotes apoptosis through the p53/p21 and mek-erk pathways in human esophageal squamous cell carcinoma. Oncol. Rep. 2014, 32, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.C.; Zhou, X.P.; Wang, X.A.; Xu, M.D.; Chen, T.; Chen, T.Y.; Zhou, P.H.; Zhang, Y.Q. Cordycepin induces apoptosis and g2/m phase arrest through the erk pathways in esophageal cancer cells. J. Cancer 2019, 10, 2415–2424. [Google Scholar]
- Cheng, A.C.; Hsu, Y.C.; Tsai, C.C. The effects of cucurbitacin e on gadd45β-trigger g2/m arrest and jnk-independent pathway in brain cancer cells. J. Cell. Mol. Med. 2019, 23, 3512–3519. [Google Scholar]
- Burgess, D.J. Apoptosis: Refined and lethal. Nat. Rev. Cancer 2013, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, K.C.; Bonzon, C.; Green, D.R. The machinery of programmed cell death. Pharmacol. Ther. 2001, 92, 57–70. [Google Scholar] [CrossRef]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Bba-Mol. Cell Res. 2013, 1833, 3448–3459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberg-Lerner, A.; Bialik, S.; Simon, H.U.; Kimchi, A. Life and death partners: Apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009, 16, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Venkatarame Gowda Saralamma, V.; Kim, S.; Ha, S.; Raha, S.; Lee, W.; Kim, E.; Lee, S.; Heo, J.; Kim, G. Pectolinarigenin induced cell cycle arrest, autophagy, and apoptosis in gastric cancer cell via pi3k/akt/mtor signaling pathway. Nutrients 2018, 10, 1043. [Google Scholar]
- Wang, G.; Zhang, T.; Sun, W.; Wang, H.; Yin, F.; Wang, Z.; Zuo, D.; Sun, M.; Zhou, Z.; Lin, B.; et al. Arsenic sulfide induces apoptosis and autophagy through the activation of ros/jnk and suppression of akt/mtor signaling pathways in osteosarcoma. Free Radic Biol. Med. 2017, 106, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zhang, C.; Huang, M.Y.; Li, W.Y.; Hu, G.Q. Cinobufagin induces autophagy-mediated cell death in human osteosarcoma u2os cells through the ros/jnk/p38 signaling pathway. Oncol. Rep. 2016, 36, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Son, K.M.; Kim, K.Y.; Yu, S.N.; Park, S.G.; Kim, Y.W.; Nam, H.W.; Suh, J.T.; Ji, J.H.; Ahn, S.C. Deoxypodophyllotoxin induces cytoprotective autophagy against apoptosis via inhibition of pi3k/akt/mtor pathway in osteosarcoma u2os cells. Pharm. Rep. 2017, 69, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.A.; Kiezun, A.; Tonzi, P.; Van Allen, E.M.; Carter, S.L.; Baca, S.C.; Cowley, G.S.; Bhatt, A.S.; Rheinbay, E.; Pedamallu, C.S.; et al. Complementary genomic approaches highlight the pi3k/mtor pathway as a common vulnerability in osteosarcoma. Proc. Natl. Acad. Sci. USA 2014, 111, E5564–E5573. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Guan, K.L. Mtor: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Saiki, S.; Sasazawa, Y.; Imamichi, Y.; Kawajiri, S.; Fujimaki, T.; Tanida, I.; Kobayashi, H.; Sato, F.; Sato, S.; Ishikawa, K.; et al. Caffeine induces apoptosis by enhancement of autophagy via pi3k/akt/mtor/p70s6k inhibition. Autophagy 2011, 7, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, B.S.; Liu, L.; Luo, Y.; Yin, J.; Zhou, H.Y.; Chen, W.X.; Shen, T.; Han, X.Z.; Huang, S.L. Hydrogen peroxide inhibits mtor signaling by activation of ampk alpha leading to apoptosis of neuronal cells. Lab. Investig. 2010, 90, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. Mtor signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Kliosnky, D. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) (vol 12, pg 1, 2015). Autophagy 2016, 12, 1–222. [Google Scholar]
- Buiatti, E.; Palli, D.; Decarli, A.; Amadori, D.; Avellini, C.; Bianchi, S.; Biserni, R.; Cipriani, F.; Cocco, P.; Giacosa, A.; et al. A case-control study of gastric cancer and diet in italy. Int. J. Cancer 1989, 44, 611–616. [Google Scholar] [CrossRef]
- Sundaram, S.G.; Milner, J.A. Diallyl disulfide inhibits the proliferation of human tumor cells in culture. Biochim. Biophys. Acta 1996, 1315, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Kwon, K.B.; Yoo, S.J.; Ryu, D.G.; Yang, J.Y.; Rho, H.W.; Kim, J.S.; Park, J.W.; Kim, H.R.; Park, B.H. Induction of apoptosis by diallyl disulfide through activation of caspase-3 in human leukemia hl-60 cells. Biochem. Pharm. 2002, 63, 41–47. [Google Scholar] [CrossRef]
- Knowles, L.M.; Milner, J.A. Depressed p34(cdc2) kinase activity and g(2)/m phase arrest induced by diallyl disulfide in hct-15 cells. Nutr. Cancer 1998, 30, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Kapinova, A.; Stefanicka, P.; Kubatka, P.; Zubor, P.; Uramova, S.; Kello, M.; Mojzis, J.; Blahutova, D.; Qaradakhi, T.; Zulli, A.; et al. Are plant-based functional foods better choice against cancer than single phytochemicals? A critical review of current breast cancer research. Biomed. Pharmacother. = Biomed. Pharmacother. 2017, 96, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Kubatka, P.; Caprnda, M.; Varghese, E.; Zolakova, B.; Zubor, P.; Opatrilova, R.; Kruzliak, P.; Stefanicka, P.; Büsselberg, D. Chemotherapeutic agents for the treatment of metastatic breast cancer: An update. Biomed. Pharmacother. = Biomed. Pharmacother. 2018, 101, 458–477. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kang, S.; Kim, D.Y.; You, S.; Park, D.; Oh, S.C.; Lee, D.H. Diallyl disulfide (dads) boosts trail-mediated apoptosis in colorectal cancer cells by inhibiting bcl-2. Food Chem. Toxicol.: Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 125, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, Y.; Zhou, H.; Zhu, J.; Tong, Z.; Qin, S.; Liu, D. Organosulfur compounds induce cytoprotective autophagy against apoptosis by inhibiting mtor phosphorylation activity in macrophages. Acta Biochim. Et Biophys. Sin. 2018, 50, 1085–1093. [Google Scholar] [CrossRef]
- Budihardjo, I.; Oliver, H.; Lutter, M.; Luo, X.; Wang, X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999, 15, 269–290. [Google Scholar] [CrossRef]
- Kroemer, G. The proto-oncogene bcl-2 and its role in regulating apoptosis. Nat. Med. 1997, 3, 614–620. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, F. Research advances in selective adaptor protein autophagy of p62/sequestosome-1. Chin. J. Pharmacol. Toxicol. 2016, 30, 258–265. [Google Scholar]
- Yu, L.; Alva, A.; Su, H.; Dutt, P.; Freundt, E.; Welsh, S.; Baehrecke, E.H.; Lenardo, M.J. Regulation of an atg7-beclin 1 program of autophagic cell death by caspase-8. Science 2004, 304, 1500–1502. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Shankar, S.; Srivastava, R.K. Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via pi3k/akt/mtor signaling pathway. Cancer Lett. 2014, 343, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific pi3k signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Z.; Guan, X.; Chao, R.; Huang, C.; Li, D.; Yang, P.; Liu, S.; Hasegawa, T.; Guo, J.; Li, M. Diallyl Disulfide Induces Apoptosis and Autophagy in Human Osteosarcoma MG-63 Cells through the PI3K/Akt/mTOR Pathway. Molecules 2019, 24, 2665. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24142665
Yue Z, Guan X, Chao R, Huang C, Li D, Yang P, Liu S, Hasegawa T, Guo J, Li M. Diallyl Disulfide Induces Apoptosis and Autophagy in Human Osteosarcoma MG-63 Cells through the PI3K/Akt/mTOR Pathway. Molecules. 2019; 24(14):2665. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24142665
Chicago/Turabian StyleYue, Ziqi, Xin Guan, Rui Chao, Cancan Huang, Dongfang Li, Panpan Yang, Shanshan Liu, Tomoka Hasegawa, Jie Guo, and Minqi Li. 2019. "Diallyl Disulfide Induces Apoptosis and Autophagy in Human Osteosarcoma MG-63 Cells through the PI3K/Akt/mTOR Pathway" Molecules 24, no. 14: 2665. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24142665