A Reversible Phase Transition of 2D Coordination Layers by B–H∙∙∙Cu(II) Interactions in a Coordination Polymer
Abstract
:1. Introduction
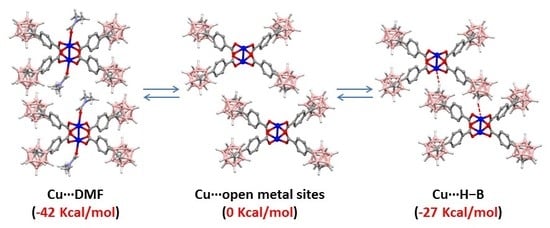
2. Results and Discussion
3. Materials and Methods
3.1. Characterization and Methods
3.2. Materials
3.3. Crystallography
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S.-i. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Liu, X.-Q.; Jiang, H.-L.; Sun, L.-B. Metal-organic frameworks for heterogeneous basic catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-S.; Lu, Y.; Chen, K.; Zhao, Y.; Wang, P.; Sun, W.-Y. Metal-organic frameworks with catalytic centers: From synthesis to catalytic application. Coord. Chem. Rev. 2019, 378, 262–280. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.; Preuss, K.; Titirici, M.-M.; Rodríguez-Reinoso, F. Nanoporous materials for the onboard storage of natural gas. Chem. Rev. 2017, 117, 1796–1825. [Google Scholar] [CrossRef]
- Bobbitt, N.S.; Mendonca, M.L.; Howarth, A.J.; Islamoglu, T.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Metal-organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents. Chem. Soc. Rev. 2017, 46, 3357–3385. [Google Scholar] [CrossRef]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xie, L.-H.; Li, J.-R.; Ma, Y.; Seminario, J.M.; Balbuena, P.B. CO2 Capture and separations using MOFs: Computational and experimental studies. Chem. Rev. 2017, 117, 9674–9754. [Google Scholar] [CrossRef] [PubMed]
- Gassensmith, J.J.; Kim, J.Y.; Holcroft, J.M.; Farha, O.K.; Stoddart, J.F.; Hupp, J.T.; Jeong, N.C. A metal-organic framework-based material for electrochemical sensing of carbon dioxide. J. Am. Chem. Soc. 2014, 136, 8277–8282. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-W.; Wei, Y.-S.; Dong, X.-Y.; Wu, X.-H.; Du, C.-X.; Zang, S.-Q.; Mak, T.C.W. Hypersensitive dual-function luminescence switching of a silver-chalcogenolate cluster-based metal-organic framework. Nat. Chem. 2017, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- Easun, T.L.; Moreau, F.; Yan, Y.; Yang, S.; Schröder, M. Structural and dynamic studies of substrate binding in porous metal-organic frameworks. Chem. Soc. Rev. 2017, 46, 239–274. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E.; Brammer, L. Coordination change, lability and hemilability in metal-organic frameworks. Chem. Soc. Rev. 2017, 46, 5444–5462. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Vlaisavljevich, B.; Britt, D.K.; Brown, C.M.; Haranczyk, M.; Neaton, J.B.; Smit, B.; Long, J.R.; Queen, W.L. Understanding small-molecule interactions in metal-organic frameworks: Coupling experiment with theory. Adv. Mater. 2015, 27, 5785–5796. [Google Scholar] [CrossRef]
- Furukawa, H.; Kim, J.; Ockwig, N.W.; O’Keeffe, M.; Yaghi, O.M. Control of vertex geometry, structure dimensionality, functionality, and pore metrics in the reticular synthesis of crystalline metal-organic frameworks and polyhedra. J. Am. Chem. Soc. 2008, 130, 11650–11661. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Vodak, D.; Sudik, A.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Geometric requirements and examples of important structures in the assembly of square building blocks. Proc. Natl. Acad. Sci. USA 2002, 99, 4900–4904. [Google Scholar] [CrossRef] [Green Version]
- Köberl, M.; Cokoja, M.; Herrmann, W.A.; Kühn, F.E. From molecules to materials: Molecular paddle-wheel synthons of macromolecules, cage compounds and metal-organic frameworks. Dalton Trans. 2011, 40, 6834–6859. [Google Scholar] [CrossRef]
- Howarth, A.J.; Peters, A.W.; Vermeulen, N.A.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Best practices for the synthesis, activation, and characterization of metal-organic frameworks. Chem. Mater. 2017, 29, 26–39. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Karagiaridi, O.; Farha, O.K.; Hupp, J.T. Activation of metal-organic framework materials. Cryst. Eng. Commun. 2013, 15, 9258–9264. [Google Scholar] [CrossRef]
- Choi, J.S.; Bae, J.; Lee, E.J.; Jeong, N.C. A chemical role for trichloromethane: Room-temperature removal of coordinated solvents from open metal sites in the copper-based metal-organic frameworks. Inorg. Chem. 2018, 57, 5225–5231. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Lee, E.J.; Jeong, N.C. Metal coordination and metal activation abilities of commonly unreactive chloromethanes toward metal-organic frameworks. Chem. Commun. 2018, 54, 6458–6471. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Yun, W.S.; Kim, M.-B.; Kim, J.Y.; Bae, Y.-S.; Lee, J.; Jeong, N.C. A chemical route to activation of open metal sites in the copper-based metal-organic framework materials HKUST-1 and Cu-MOF-2. J. Am. Chem. Soc. 2015, 137, 10009–10015. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Choi, J.S.; Hwang, S.; Yun, W.S.; Song, D.; Lee, J.; Jeong, N.C. Multiple coordination exchanges for room-temperature activation of open-metal sites in metal-organic frameworks. ACS Appl. Mater. Interfaces 2017, 9, 24743–24752. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ledwaba, M.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S.; Pang, W. Structural defects in metal-organic frameworks (MOFs): Formation, detection and control towards practices of interests. Coord. Chem. Rev. 2017, 349, 169–197. [Google Scholar] [CrossRef]
- Scholz, M.; Hey-Hawkins, E. Carbaboranes as pharmacophores: Properties, synthesis, and application strategies. Chem. Rev. 2011, 111, 7035–7062. [Google Scholar] [CrossRef]
- Grimes, R.N. Carboranes. In Carboranes, 3rd ed.; Academic Press: Oxford, UK, 2016. [Google Scholar] [Green Version]
- Teixidor, F.; Viñas, C. Science of Synthesis; Thieme: Stuttgart, Germany, 2005; Volume 6, pp. 1275–1325. [Google Scholar]
- Teixidor, F.; Barberà, G.; Vaca, A.; Kivekäs, R.; Sillanpää, R.; Oliva, J.; Viñas, C. Are methyl groups electron-donating or electron-withdrawing in boron clusters? Permethylation of o-carborane. J. Am. Chem. Soc. 2005, 127, 10158–10159. [Google Scholar] [CrossRef]
- Poater, J.; Solà, M.; Viñas, C.; Teixidor, F. π Aromaticity and three-dimensional aromaticity: Two sides of the same coin. Angew. Chem. Int. Ed. 2014, 53, 12191–12195. [Google Scholar] [CrossRef]
- Fontanet, M.; Popescu, A.R.; Fontrodona, X.; Rodríguez, M.; Romero, I.; Teixidor, F.; Viñas, C.; Aliaga-Alcalde, N.; Ruiz, E. Design of dinuclear copper species with carboranylcarboxylate ligands: Study of their steric and electronic effects. Chem. Eur. J. 2011, 17, 13217–13229. [Google Scholar] [CrossRef] [PubMed]
- Fontanet, M.; Rodríguez, M.; Romero, I.; Fontrodona, X.; Teixidor, F.; Viñas, C.; Aliaga-Alcalde, N.; Matějíček, P. A water soluble Mn(ii) polymer with aqua metal bridges. Dalton Trans. 2013, 42, 7838–7841. [Google Scholar] [CrossRef] [PubMed]
- Fontanet, M.; Rodríguez, M.; Fontrodona, X.; Romero, I.; Teixidor, F.; Viñas, C.; Aliaga-Alcalde, N.; Matějíček, P. Water-soluble manganese inorganic polymers: The role of carborane clusters and producing large structural adjustments from minor molecular changes. Chem. Eur. J. 2014, 20, 13993–14003. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Spokoyny, A.M.; Farha, O.K.; Snurr, R.Q.; Hupp, J.T.; Mirkin, C.A. Separation of gas mixtures using Co(II) carborane-based porous coordination polymers. Chem. Commun. 2010, 46, 3478–3480. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-S.; Farha, O.K.; Spokoyny, A.M.; Mirkin, C.A.; Hupp, J.T.; Snurr, R.Q. Carborane-based metal-organic frameworks as highly selective sorbents for CO2 over methane. Chem. Commun. 2008. [Google Scholar] [CrossRef] [PubMed]
- Clingerman, D.J.; Morris, W.; Mondloch, J.E.; Kennedy, R.D.; Sarjeant, A.A.; Stern, C.; Hupp, J.T.; Farha, O.K.; Mirkin, C.A. Stabilization of a highly porous metal-organic framework utilizing a carborane-based linker. Chem. Commun. 2015, 51, 6521–6523. [Google Scholar] [CrossRef]
- Kennedy, R.D.; Krungleviciute, V.; Clingerman, D.J.; Mondloch, J.E.; Peng, Y.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Hupp, J.T.; Yildirim, T.; et al. Carborane-based metal-organic framework with high methane and hydrogen storage capacities. Chem. Mater. 2013, 25, 3539–3543. [Google Scholar] [CrossRef]
- Spokoyny, A.M.; Farha, O.K.; Mulfort, K.L.; Hupp, J.T.; Mirkin, C.A. Porosity tuning of carborane-based metal-organic frameworks (MOFs) via coordination chemistry and ligand design. Inorg. Chim. Acta 2010, 364, 266–271. [Google Scholar] [CrossRef]
- Farha, O.K.; Spokoyny, A.M.; Mulfort, K.L.; Hawthorne, M.F.; Mirkin, C.A.; Hupp, J.T. Synthesis and hydrogen sorption properties of carborane based metal-organic framework materials. J. Am. Chem. Soc. 2007, 129, 12680–12681. [Google Scholar] [CrossRef]
- Farha, O.K.; Spokoyny, A.M.; Mulfort, K.L.; Galli, S.; Hupp, J.T.; Mirkin, C.A. Gas-sorption properties of cobalt(II)-carborane-based coordination polymers as a function of morphology. Small 2009, 5, 1727–1731. [Google Scholar] [CrossRef]
- Huang, S.L.; Lin, Y.J.; Yu, W.B.; Jin, G.X. Porous frameworks based on carborane-Ln 2(CO 2) 6: Architecture influenced by lanthanide contraction and selective CO2 capture. Chem. Plus Chem. 2012, 77, 141–147. [Google Scholar]
- Huang, S.L.; Weng, L.H.; Jin, G.X. Bottom-up synthesis of coordination polymers based on carborane backbones and Cu2(CO2)4 paddle-wheel: Ligand metathesis with metallotecons. Dalton Trans. 2012, 41, 11657–11662. [Google Scholar] [CrossRef] [PubMed]
- Boldog, I.; Bereciartua, P.J.; Bulánek, R.; Kučeráková, M.; Tomandlová, M.; Dušek, M.; Macháček, J.; De Vos, D.; Baše, T. 10-Vertex closo-carborane: A unique ligand platform for porous coordination polymers. Cryst. Eng. Comm. 2016, 18, 2036–2040. [Google Scholar] [CrossRef]
- Tsang, M.Y.; Rodríguez-Hermida, S.; Stylianou, K.C.; Tan, F.; Negi, D.; Teixidor, F.; Viñas, C.; Choquesillo-Lazarte, D.; Verdugo-Escamilla, C.; Guerrero, M.; et al. Carborane bis-pyridylalcohols as linkers for coordination polymers: Synthesis, crystal structures, and guest-framework dependent mechanical properties. Cryst. Growth Des. 2017, 17, 846–857. [Google Scholar] [CrossRef]
- Rodriguez-Hermida, S.; Tsang, M.Y.; Vignatti, C.; Stylianou, K.C.; Guillerm, V.; Perez-Carvajal, J.; Teixidor, F.; Vinas, C.; Choquesillo-Lazarte, D.; Verdugo-Escamilla, C.; et al. Switchable surface hydrophobicity-hydrophilicity of a metal-organic framework. Angew. Chem. Int. Ed. Engl. 2016, 55, 16049–16053. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; López-Periago, A.; Light, M.E.; Cirera, J.; Ruiz, E.; Borrás, A.; Teixidor, F.; Viñas, C.; Domingo, C.; Planas, J.G. An unprecedented stimuli-controlled single-crystal reversible phase transition of a metal–organic framework and its application to a novel method of guest encapsulation. Adv. Mater. 2018, 30, 1800726. [Google Scholar] [CrossRef]
- Di Salvo, F.; Camargo, B.; Garcia, Y.; Teixidor, F.; Viñas, C.; Giner Planas, J.; Light, M.E.; Hursthouse, M.B. Supramolecular architectures in o-carboranyl alcohols bearing N-aromatic rings: Syntheses, crystal structures and melting points correlation. Crysteng. Comm. 2011, 13, 5788–5806. [Google Scholar] [CrossRef]
- Planas, J.G.; Vinas, C.; Teixidor, F.; Comas-Vives, A.; Ujaque, G.; Lledos, A.; Light, M.E.; Hursthouse, M.B. Self-assembly of mercaptane-metallacarborane complexes by an unconventional cooperative effect: A C-H center dot center dot center dot S-H center dot center dot center dot H-B hydrogen/dihydrogen bond interaction. J. Am. Chem. Soc. 2005, 127, 15976–15982. [Google Scholar] [CrossRef]
- Fox, M.A.; Hughes, A.K. Cage CH⋯X interactions in solid-state structures of icosahedral carboranes. Coord. Chem. Rev. 2004, 248, 457–476. [Google Scholar] [CrossRef]
- Chaari, M.; Kelemen, Z.; Planas, J.G.; Teixidor, F.; Choquesillo-Lazarte, D.; Ben Salah, A.; Viñas, C.; Núñez, R. Photoluminescence in m-carborane–anthracene triads: A combined experimental and computational study. J. Mater. Chem. C 2018, 6, 11336–11347. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, H.; Yan, H.; Zou, W.; Cremer, D. B–H···π Interaction: A new type of nonclassical hydrogen bonding. J. Am. Chem. Soc. 2016, 138, 4334–4337. [Google Scholar] [CrossRef]
- Terrasson, V.; Garcia, Y.; Farras, P.; Teixidor, F.; Viñas, C.; Giner Planas, J.; Prim, D.; Light, M.E.; Hursthouse, M.B. Crystal engineering of o-carboranyl alcohols: Syntheses, crystal structures and thermal properties. Crysteng. Comm. 2010, 12, 4109–4123. [Google Scholar] [CrossRef]
- Eleazer, B.J.; Peryshkov, D.V. Coordination chemistry of carborane clusters: Metal-boron bonds in carborane, carboranyl, and carboryne complexes. Comments Inorg. Chem. 2018, 38, 79–109. [Google Scholar] [CrossRef]
- Riley, L.E.; Chan, A.P.Y.; Taylor, J.; Man, W.Y.; Ellis, D.; Rosair, G.M.; Welch, A.J.; Sivaev, I.B. Unprecedented flexibility of the 1,1′-bis(o-carborane) ligand: Catalytically-active species stabilised by B-agostic B–H⇀Ru interactions. Dalton Trans. 2016, 45, 1127–1137. [Google Scholar] [CrossRef]
- Cunha-Silva, L.; Carr, M.J.; Kennedy, J.D.; Hardie, M.J. Silver-dabco coordination networks with distinct carbaborane anions: Investigating Ag···H–B and Ag···I–B interactions. Cryst. Growth Des. 2013, 13, 3162–3170. [Google Scholar] [CrossRef]
- Teixidor, F.; Flores, M.A.; Viñas, C.; Sillanpää, R.; Kivekäs, R. Exo-nido-Cyclooctadienerhodacarboranes: Synthesis, reactivity, and catalytic properties in alkene hydrogenation. J. Am. Chem. Soc. 2000, 122, 1963–1973. [Google Scholar] [CrossRef]
- Behnken, P.E.; Marder, T.B.; Baker, R.T.; Knobler, C.B.; Thompson, M.R.; Hawthorne, M.F. Synthesis, structural characterization, and stereospecificity in the formation of bimetallic rhodacarborane clusters containing rhodium-hydrogen-boron bridge interactions. J. Am. Chem. Soc. 1985, 107, 932–940. [Google Scholar] [CrossRef]
- Long, J.A.; Marder, T.B.; Behnken, P.E.; Hawthorne, M.F. Metallacarboranes in catalysis. 3. Synthesis and reactivity of exo-nido-phosphinerhodacarboranes. J. Am. Chem. Soc. 1984, 106, 2979–2989. [Google Scholar] [CrossRef]
- Knobler, C.B.; Marder, T.B.; Mizusawa, E.A.; Teller, R.G.; Long, J.A.; Behnken, P.E.; Hawthorne, M.F. Metallacarboranes in catalysis. 4. Structures of closo- and exo-nido-phosphinerhodacarboranes and a [(PPh3)3Rh]+[nido-7-R-7,8-C2B9H11]- salt. J. Am. Chem. Soc. 1984, 106, 2990–3004. [Google Scholar] [CrossRef]
- Teixidor, F.; Flores, M.A.; Viñas, C.; Kivekäs, R.; Sillanpää, R. [Rh(7-SPh-8-Me-7,8-C2B9H10)(PPh3)2]: A new rhodacarborane with enhanced activity in the hydrogenation of 1-Alkenes. Angew. Chem. Int. Ed. Engl. 1996, 35, 2251–2253. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, S.-T.; Cui, P.-F.; Aznarez, F.; Jin, G.-X. Iridium-induced regioselective B–H and C–H activations at azo-substituted m-carboranes: Facile access to polynuclear complexes. Chem. Commun. 2019, 55, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.-J.; Deng, W. Half-sandwich late transition metal complexes based on functionalized carborane ligands. Coord. Chem. Rev. 2016, 309, 21–35. [Google Scholar] [CrossRef]
- Vinas, C.; Nunez, R.; Flores, M.A.; Teixidor, F.; Kivekas, R.; Sillanpaea, R. Agostic B-H.fwdharw.Ru Bonds in exo-Monophosphino-7,8-dicarba-nido-undecaborate derivatives. Organometallics 1995, 14, 3952–3957. [Google Scholar] [CrossRef]
- Viñas, C.; Nuñez, R.; Teixidor, F.; Kivekäs, R.; Sillanpää, R. Modulation of agostic B−H⇀Ru bonds in exo-Monophosphino-7,8-Dicarba-nido-undecaborate derivatives. Organometallics 1996, 15, 3850–3858. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Wang, L.; Duttwyler, S.; Xing, H. A microporous metalo-rganic framework supramolecularly assembled from a Cu(II) dodecaborate cluster complex for selective gas separation. Angew. Chem. Int. Ed. 2019. [Google Scholar]
- Zhu, C.; Mao, Q.; Li, D.; Li, C.; Zhou, Y.; Wu, X.; Luo, Y.; Li, Y. A readily available urea based MOF that act as a highly active heterogeneous catalyst for Friedel-Crafts reaction of indoles and nitrostryenes. Catal. Commun. 2018, 104, 123–127. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, B.; Li, M.; Zhao, M.-J.; Xie, Y.-B.; Li, J.-R. Different two-dimensional metal-organic frameworks through ligand modification. J. Coord. Chem. 2016, 69, 2193–2199. [Google Scholar] [CrossRef]
- He, Z.; Pang, Q.; Rankine, D.; Sumby, C.J.; Zhang, L.; Doonan, C.J.; Li, Q. Encapsulation of polyoxometalates within layered metal–organic frameworks with topological and pore control. Cryst. Eng. Comm. 2013, 15, 9340–9343. [Google Scholar] [CrossRef]
- Sasaki, T.; Hisaki, I.; Miyano, T.; Tohnai, N.; Morimoto, K.; Sato, H.; Tsuzuki, S.; Miyata, M. Linkage control between molecular and supramolecular chirality in 21-helical hydrogen-bonded networks using achiral components. Nat. Commun. 2013, 4, 1787. [Google Scholar] [CrossRef]
- Zhong, R.-Q.; Zou, R.-Q.; Xu, Q. Solvent-induced deviation in square-grid layers of microporous Cu(ii) isophthalates: Layer stacking and gas adsorption properties. Cryst. Eng. Comm. 2011, 13, 577–584. [Google Scholar] [CrossRef]
- Note that it was not possible to differentiate the Cu-coordinated solvent molecules by elemental analysis in this case. Thus, the formula could be either [Cu(L1)2(H2O)(MeOH)]∙MeOH or [Cu(L1)2(MeOH)2] H2O.
- Wang, X.-F.; Zhang, Y.-B.; Huang, H.; Zhang, J.-P.; Chen, X.-M. Microwave-assisted solvothermal synthesis of a dynamic porous metal-carboxylate framework. Cryst. Growth Des. 2008, 8, 4559–4563. [Google Scholar] [CrossRef]
- Hicken, A.; White, A.J.P.; Crimmin, M.R. Reversible Coordination of Boron–, Aluminum–, Zinc–, Magnesium–, and Calcium–Hydrogen bonds to bent {CuL2} fragments: Heavy σ complexes of the lightest coinage metal. Inorg. Chem. 2017, 56, 8669–8682. [Google Scholar] [CrossRef] [PubMed]
- Biradha, K.; Hongo, Y.; Fujita, M. Crystal-to-crystal sliding of 2D coordination layers triggered by guest exchange. Angew. Chem. Int. Ed. 2002, 41, 3395–3398. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Liao, P.-Q.; Zhou, H.-L.; Lin, R.-B.; Chen, X.-M. Single-crystal X-ray diffraction studies on structural transformations of porous coordination polymers. Chem. Soc. Rev. 2014, 43, 5789–5814. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.A. Icosahedral Carborane Derivatives. Ph.D. Thesis, Durham University, Durham, UK, 1991. [Google Scholar]
- Bruker. APEX3 Software, V2016.1; Bruker AXS Inc.: Madison, WI, USA, 2016.
- Sheldrick, G.M. TWINABS; University of Goöttingen: Goöttingen, Germany, 2012. [Google Scholar]
- Sheldrick, G. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Spek, A. Structure validation in chemical crystallography. Acta Cryst. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Gaussian 09; Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013.
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Ehlers, A.W.; Böhme, M.; Dapprich, S.; Gobbi, A.; Höllwarth, A.; Jonas, V.; Köhler, K.F.; Stegmann, R.; Veldkamp, A.; Frenking, G. A set of f-polarization functions for pseudo-potential basis sets of the transition metals ScCu, YAg and LaAu. Chem. Phys. Lett. 1993, 208, 111–114. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Rodríguez-Fortea, A.; Alemany, P.; Alvarez, S.; Ruiz, E. Exchange coupling in carboxylato-bridged dinuclear copper (II) compounds: A density functional study. Chem. Eur. J. 2001, 7, 627–637. [Google Scholar] [CrossRef]
- Ali, M.E.; Datta, S.N. Theoretical investigation of magnetic properties of a dinuclear copper complex [Cu2(μ-OAc)4(MeNHpy)2]. J. Mol. Struct. 2006, 775, 19–27. [Google Scholar] [CrossRef]
- Bureekaew, S.; Amirjalayer, S.; Schmid, R. Orbital directing effects in copper and zinc based paddle-wheel metal organic frameworks: The origin of flexibility. J. Mater. Chem. 2012, 22, 10249–10254. [Google Scholar] [CrossRef]
- Tafipolsky, M.; Amirjalayer, S.; Schmid, R. First-principles-derived force field for copper paddle-wheel-based metal-organic frameworks. J. Phys. Chem. C 2010, 114, 14402–14409. [Google Scholar] [CrossRef]
- Alzahrani, K.A.H.; Deeth, R.J. Density functional calculations reveal a flexible version of the copper paddlewheel unit: Implications for metal organic frameworks. Dalton Trans. 2016, 45, 11944–11948. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |






| Coordination Polymer | SGa | Db | 44 nets Diagonal Distances (Å)c | P (Å)d | Layer Thicknesse (Å) | Interlayer Distances (Å) |
|---|---|---|---|---|---|---|
| 1-DMA (this work) | P-1 | 1.262 | 16.2 × 29.0 | 16.2 | 15.41 | 6.94 |
| 1-DMF (this work) | P-1 | 1.355 | 14.4 × 29.4 | 14.4 | 14.95 | 6.86 |
| 1-MeOH (this work) | P21/c | 1.367 | 12.5 × 29.8 | 12.5 | 16.29 | 7.06 |
| PELFOEf ([CuL1∙DMA]DMF) | C2/c | 1.448 | 19.7 × 27.6 | 19.7 | - | interpenetrated |
| MAPLATf ([CuL1∙DMA]H2O) | C2/c | 1.353 | 19.9 × 27.6 | 19.9 | - | interpenetrated |
| OFEHOYf ([CuL2∙DMF]DMF) | C2/c | 1.245 | 19.6 × 27.7 | 19.6 | - | interpenetrated |
| NEYRIUf ([CuL3∙MeOH]MeOH) | C2/c | 1.261 | 12.8 × 13.8 | 12.8 | 10.313 | 10.187 |
| NEYREQf ([CuL3∙MeOH]DMF) | P21/n | 12.7 × 14.0 | 12.7 | 9.827 | 10.454 |
| Model Compounds (Molecules at the Apical Positions) | Energya (Kcal/mol) | Cu-Cu Distances (Å) | Vibrational Cu-Cu Frequencies (cm−1) | ||
|---|---|---|---|---|---|
| Exp. | Calc. | Exp. | Calc. | ||
 (2 Molecules of DMF) | −41.6 | 2.641b | 2.668 | 162b | 207 |
 (2 Molecules of MeOH) | −41.0 | 2.620c | 2.597 | - | 194 |
 (None) | 0 | - | 2.507 | - | 223 |
 (2 Molecules of m-Carborane) | −26.7 | - | 2.561 | - | 206 |
 (1 Molecule of m-Carborane) | −13.6 | - | 2.536 | - | 214 |
 (1 Molecule of m-Carborane 1 molecule of H2O) | −34.4 | - | 2.577 | - | 192 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, L.; Fonquernie, P.G.; Light, M.E.; Norjmaa, G.; Ujaque, G.; Choquesillo-Lazarte, D.; Fraile, J.; Teixidor, F.; Viñas, C.; Planas, J.G. A Reversible Phase Transition of 2D Coordination Layers by B–H∙∙∙Cu(II) Interactions in a Coordination Polymer. Molecules 2019, 24, 3204. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24173204
Gan L, Fonquernie PG, Light ME, Norjmaa G, Ujaque G, Choquesillo-Lazarte D, Fraile J, Teixidor F, Viñas C, Planas JG. A Reversible Phase Transition of 2D Coordination Layers by B–H∙∙∙Cu(II) Interactions in a Coordination Polymer. Molecules. 2019; 24(17):3204. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24173204
Chicago/Turabian StyleGan, Lei, Pol G. Fonquernie, Mark E. Light, Gantulga Norjmaa, Gregori Ujaque, Duane Choquesillo-Lazarte, Julio Fraile, Francesc Teixidor, Clara Viñas, and José G. Planas. 2019. "A Reversible Phase Transition of 2D Coordination Layers by B–H∙∙∙Cu(II) Interactions in a Coordination Polymer" Molecules 24, no. 17: 3204. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24173204