The Thioredoxin System is Regulated by the ASK-1/JNK/p38/Survivin Pathway During Germ Cell Apoptosis
Abstract
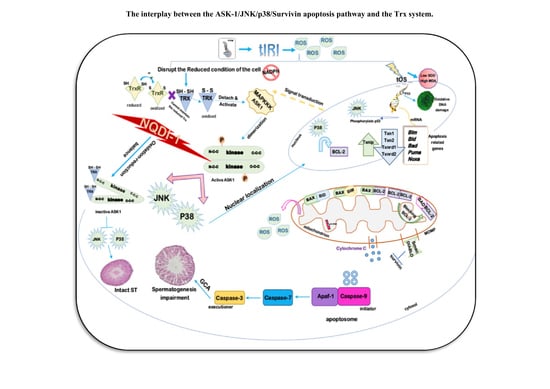
:1. Introduction
2. Results
2.1. NQDI-1 Protects against Damge to Testicular Histology and Spermatogenesis
2.2. NQDI-1 Inhibits Testicular Oxidative Stress
2.3. NQDI-1 Prevents Germ Cell Apoptosis
2.4. NQDI-1 Regulates the Expression of the Trx System
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Drug
4.3. Animals and Surgical Procedure
4.4. RNA Isolation, Reverse Transcription (RT) and Real-Time PCR
4.5. Biochemical Analyses
4.6. Histological Examination
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| AP-1 | Activator Protein-1 |
| ASK-1 | Apoptosis Signal regulating Kinase-1 |
| BAD | Bcl2 associated agonist of cell death |
| BAEC | Bovine Aorta Endothelial Cells |
| BAX | Bcl2 associated X |
| BCL-2 | B-cell lymphoma 2 |
| BID | BH3 interacting domain |
| Birc5 | Baculoviral IAP repeat containing 5 |
| BSA | Bovine Serum Albumin |
| cDNA | Complementary deoxyribonucleic acid |
| cGMP | Cyclic Guanosine mono phosphate |
| DAB | Chromogen 3,3′-diaminobenzidine |
| DAPI | 4′,6 diamidino-2-phenyl indole |
| DNA | Deoxyribonucleic acid |
| DPX | Distyrene Plasticizer Xylene |
| ELISA | Enzyme-linked immunosorbent assay |
| GCA | Germ Cell Apoptosis |
| GSH | Glutathione |
| i.p. | Intraperitoneally |
| IAP | Inhibitor of Apoptosis |
| ICLAS | International Council for Laboratory Animal Sciences |
| JNK | c-jun N-terminal kinase |
| MAPK | Mitogen Activated Protein Kinase |
| MDA | Malondialdehyde |
| MPP+ | 1-methyl-4-phenylpyridinum |
| NADP+/NADPH | Nicotinamide adenine dinucleotide phosphate |
| NQDI-1 | Ethyl 2,7-dioxo-2,7-dihydro-3H-naphtho[1,2,3-de]quinoline-1-carboxylate |
| OS | Oxidative Stress |
| PBS | Phosphate buffer solution |
| PCR | Polymerase Chain Reaction |
| ph-JNK | Phosphorylated JNK |
| ph-p38 | Phosphorylated p38 |
| RIPA | Radioimmunoprecipitation assay |
| RNA | Ribonucleic acid |
| ROS | Reactive Oxygen Species |
| RT | Reverse Transcription |
| SD | Sprague-Dawley |
| SD | Standard Deviation |
| SOD | Superoxide dismutase |
| ST | Seminiferous Tubule |
| TD | Testicular Detorsion |
| TFs | Transcription Factors |
| tIRI | Testicular Ischemia Reperfusion Injury |
| TRX | Thioredoxin |
| TrxR | Thioredoxin Reductase |
| TrxR-1 | Thioredoxin Reductase-1 |
| TrxR-2 | Thioredoxin Reductase-2 |
| TT | Testicular Torsion |
| TTD | Testicular Torsion and Detrosion |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP Nick End Labeling |
| TXNIP | Thioredoxin Interacting Protein |
References
- Sharp, V.J.; Kieran, K.; Arlen, A.M. Testicular torsion: Diagnosis, evaluation, and management. Am. Fam. Physician 2013, 88, 835–840. [Google Scholar]
- Filho, D.W.; Torres, M.A.; Bordin, A.L.; Crezcynski-Pasa, T.B.; Boveris, A. Spermatic cord torsion, reactive oxygen and nitrogen species and ischemia-reperfusion injury. Mol. Asp. Med. 2004, 25, 199–210. [Google Scholar] [CrossRef]
- Aitken, R.J.; Jones, K.T.; Robertson, S.A. Reactive oxygen species and sperm function in sickness and in health. J. Androl. 2012, 33, 1096–1106. [Google Scholar] [CrossRef]
- Watanabe, T.; Sekine, S.; Naguro, I.; Sekine, Y.; Ichijo, H. Apoptosis Signal-regulating Kinase 1 (ASK1)-p38 Pathway-dependent Cytoplasmic Translocation of the Orphan Nuclear Receptor NR4A2 Is Required for Oxidative Stress-induced Necrosis. J. Biol. Chem. 2015, 290, 10791–10803. [Google Scholar] [CrossRef] [Green Version]
- Soga, M.; Matsuzawa, A.; Ichijo, H. Oxidative Stress-Induced Diseases via the ASK1 Signaling Pathway. Int. J. Cell Biol. 2012, 2012, 439587–439593. [Google Scholar] [CrossRef]
- Mahmood, D.F.; Abderrazak, A.; El Hadri, K.; Simmet, T.; Rouis, M. The thioredoxin system as a therapeutic target in human health and disease. Antioxid. Redox. Signal. 2013, 19, 1266–1303. [Google Scholar] [CrossRef]
- Balsera, M.; Buchanan, B.B. Evolution of the thioredoxin system as a step enabling adaptation to oxidative stress. Free Radic. Biol. Med. 2019. [Google Scholar] [CrossRef]
- Hao, H.; Li, S.; Tang, H.; Liu, B.; Cai, Y.; Shi, C.; Xiao, X. NQDI-1, an inhibitor of ASK1 attenuates acute perinatal hypoxic-ischemic cerebral injury by modulating cell death. Mol. Med. Rep. 2016, 13, 4585–4592. [Google Scholar] [CrossRef] [Green Version]
- Volynets, G.P.; Chekanov, M.O.; Synyugin, A.R.; Golub, A.G.; Kukharenko, O.P.; Bdzhola, V.G.; Yarmoluk, S.M. Identification of 3H-naphtho[1,2,3-de]quinoline-2,7-diones as inhibitors of apoptosis signal-regulating kinase 1 (ASK1). J. Med. Chem. 2011, 54, 2680–2686. [Google Scholar] [CrossRef]
- Hayakawa, R.; Hayakawa, T.; Takeda, K.; Ichijo, H. Therapeutic targets in the ASK1-dependent stress signaling pathways. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012, 88, 434–453. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Oka, S.; Masutani, H.; Nakamura, H.; Yodoi, J. The role of thioredoxin in the aging process: Involvement of oxidative stress. Antioxid. Redox. Signal. 2003, 5, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, D.T.; Emadi, E.M.A.; Tonissen, K.F.; Clarke, F.M. The thioredoxin-thioredoxin reductase system: Over-expression in human cancer. Anticancer Res. 2003, 23, 2425–2433. [Google Scholar] [PubMed]
- Urao, N.; Inomata, H.; Razvi, M.; Kim, H.W.; Wary, K.; McKinney, R.; Fukai, T.; Ushio-Fukai, M. Role of Nox2-Based NADPH Oxidase in Bone Marrow and Progenitor Cell Function Involved in Neovascularization Induced by Hindlimb Ischemia. Circ. Res. 2008, 103, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Min, W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ. Res. 2002, 90, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.; Chiueh, C.C.; Chock, P.B. Cyclic GMP-dependent protein kinase regulates the expression of thioredoxin and thioredoxin peroxidase-1 during hormesis in response to oxidative stress-induced apoptosis. J. Biol. Chem. 2003, 278, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Papaconstantinou, J. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J. 2006, 20, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Tobiume, K.; Matsuzawa, A.; Takahashi, T.; Nishitoh, H.; Morita, K.; Takeda, K.; Minowa, O.; Miyazono, K.; Noda, T.; Ichijo, H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001, 2, 222–228. [Google Scholar] [CrossRef]
- Lie, P.P.; Cheng, C.Y.; Mruk, D.D. Coordinating cellular events during spermatogenesis: A biochemical model. Trends Biochem. Sci. 2009, 34, 366–373. [Google Scholar] [CrossRef]
- Lui, W.Y.; Wong, C.H.; Mruk, D.D.; Cheng, C.Y. TGF-beta3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: An in vivo study. Endocrinology 2003, 144, 1139–1142. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, W.J.; Wang, Y.L.; Li, Q.; Yang, H.D.; Duan, Z.L.; Wang, Q. P38 participates in spermatogenesis and acrosome reaction prior to fertilization in Chinese mitten crab Eriocheir sinensis. Gene 2015, 559, 103–111. [Google Scholar] [CrossRef]
- Almog, T.; Naor, Z. The role of Mitogen activated protein kinase (MAPK) in sperm functions. Mol. Cell. Endocrinol. 2010, 314, 239–243. [Google Scholar] [CrossRef]
- Lysiak, J.J.; Nguyen, Q.A.; Kirby, J.L.; Turner, T.T. Ischemia-reperfusion of the murine testis stimulates the expression of proinflammatory cytokines and activation of c-jun N-terminal kinase in a pathway to E-selectin expression. Biol. Reprod. 2003, 69, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Baker, M.A.; Nixon, B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J. Androl. 2015, 17, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.; Mendoza, N.; Casao, A.; Pérez-Pé, R.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. c-Jun N-terminal kinase and p38 mitogen-activated protein kinase pathways link capacitation with apoptosis and seminal plasma proteins protect sperm by interfering with both routes†. Biol. Reprod. 2017, 96, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Mustacich, D.; Powis, G. Thioredoxin reductase. Biochem. J. 2000, 346, 1–8. [Google Scholar] [CrossRef]
- Bersani, N.A.; Merwin, J.R.; Lopez, N.I.; Pearson, G.D.; Merrill, G.F. Protein electrophoretic mobility shift assay to monitor redox state of thioredoxin in cells. Methods Enzymol. 2002, 347, 317–326. [Google Scholar] [PubMed]
- Liu, B.; Chen, Y.; Clair, D.K.S. ROS and p53: A versatile partnership. Free Radic. Biol. Med. 2008, 44, 1529–1535. [Google Scholar] [CrossRef] [Green Version]
- Moradi, M.N.; Karimi, J.; Khodadadi, I.; Amiri, I.; Karami, M.; Saidijam, M.; Vatannejad, A.; Tavilani, H. Evaluation of the p53 and Thioredoxin reductase in sperm from asthenozoospermic males in comparison to normozoospermic males. Free Radic. Biol. Med. 2018, 116, 123–128. [Google Scholar] [CrossRef]
- Merwin, J.R.; Mustacich, D.J.; Muller, E.G.; Pearson, G.D.; Merrill, G.F. Reporter gene transactivation by human p53 is inhibited in thioredoxin reductase null yeast by a mechanism associated with thioredoxin oxidation and independent of changes in the redox state of glutathione. Carcinogenesis 2002, 23, 1609–1615. [Google Scholar] [CrossRef]
- Agledal, L.; Niere, M.; Ziegler, M. The phosphate makes a difference: Cellular functions of NADP. Redox Rep. 2010, 15, 2–10. [Google Scholar] [CrossRef]
- Spaans, S.K.; Weusthuis, R.A.; van der Oost, J.; Kengen, S.W. NADPH-generating systems in bacteria and archaea. Front. Microbiol. 2015, 6, 742–769. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.A.; Boslett, J.; Varadharaj, S.; De Pascali, F.; Hemann, C.; Druhan, L.J.; Ambrosio, G.; El-Mahdy, M.; Zweier, J.L. Depletion of NADP(H) due to CD38 activation triggers endothelial dysfunction in the postischemic heart. Proc. Natl. Acad. Sci. USA 2015, 112, 11648–11653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; De Keulenaer, G.W.; Lee, R.T. Vitamin D(3)-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J. Biol. Chem. 2002, 277, 26496–26500. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chng, W.J. Roles of thioredoxin binding protein (TXNIP) in oxidative stress, apoptosis and cancer. Mitochondrion 2013, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.F.; Yoshioka, J. The Emerging Role of Thioredoxin-Interacting Protein in Myocardial Ischemia/Reperfusion Injury. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Cho, K.J.; Lee, S.K.; Kim, G.W. Apoptosis signal-regulating kinase 1 (Ask1) targeted small interfering RNA on ischemic neuronal cell death. Brain Res. 2011, 1412, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Saxena, G.; Chen, J.; Shalev, A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J. Biol. Chem. 2010, 285, 3997–4005. [Google Scholar] [CrossRef]
- Lane, T.; Flam, B.; Lockey, R.; Kolliputi, N. TXNIP shuttling: Missing link between oxidative stress and inflammasome activation. Front. Physiol. 2013, 4, 50–53. [Google Scholar] [CrossRef]
- Lysiak, J.J.; Turner, S.D.; Nguyen, Q.A.; Singbartl, K.; Ley, K.; Turner, T.T. Essential role of neutrophils in germ cell-specific apoptosis following ischemia/reperfusion injury of the mouse testis. Biol. Reprod. 2001, 65, 718–725. [Google Scholar] [CrossRef]
- Al-Ajmi, N.; Al-Maghrebi, M.; Renno, W.M. (-)-Epigallocatechin-3-gallate Modulates the Differential Expression of Survivin Splice Variants and Protects Spermatogenesis during Testicular Torsion. Korean J. Physiol. Pharmacol. 2013, 17, 259–265. [Google Scholar] [CrossRef]
- Liang, H.; Yu, F.; Tong, Z.; Yuan, B.; Wang, C. Effect of ischemia post-conditioning on skeletal muscle oxidative injury, mTOR, Bax, Bcl-2 proteins expression, and HIF-1α/β-actin mRNA, IL-6/β-actin mRNA and caveolin-3/β-actin mRNA expression in ischemia-reperfusion rabbits. Mol. Biol. Rep. 2013, 40, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gu, Q.; Xiang, L.; Dong, X.; Li, H.; Ni, J.; Wan, L.; Cai, G.; Chen, G. Curcumin inhibits apoptosis by modulating Bax/Bcl-2 expression and alleviates oxidative stress in testes of streptozotocin-induced diabetic rats. Ther. Clin. Risk Manag. 2017, 13, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Hatano, M.; Otaki, M.; Ogasawara, T.; Tokuhisa, T. Expression of a murine homologue of the inhibitor of apoptosis protein is related to cell proliferation. Proc. Natl. Acad. Sci. USA 1999, 96, 1457–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aitken, R.J.; Koppers, A.J. Apoptosis and DNA damage in human spermatozoa. Asian J. Androl. 2011, 13, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Clair, D.K.S. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhang, H.; Iles, K.E.; Rinna, A.; Merrill, G.; Yodoi, J.; Torres, M.; Forman, H.J. The ADP-stimulated NADPH oxidase activates the ASK-1/MKK4/JNK pathway in alveolar macrophages. Free Radic. Res. 2006, 40, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Hales, B.F. Activator protein-1 (AP-1) DNA binding activity is induced by hydroxyurea in organogenesis stage mouse embryos. Toxicol. Sci. 2005, 85, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Oberley, T.D.; Chaiswing, L.; Lin, S.M.; Epstein, C.J.; Huang, T.T.; Clair, D.S. Manganese superoxide dismutase deficiency enhances cell turnover via tumor promoter-induced alterations in AP-1 and p53-mediated pathways in a skin cancer model. Oncogene 2002, 21, 3836–3846. [Google Scholar] [CrossRef] [Green Version]
- El Eter, E. NQDI 1, an inhibitor of ASK1 attenuates acute ischemic renal injury by modulating oxidative stress and cell death. Cardiovasc. Hematol. Agents Med. Chem. 2013, 11, 179–186. [Google Scholar] [CrossRef]
- Al-Maghrebi, M.; Renno, W.M. Altered expression profile of glycolytic enzymes during testicular ischemia reperfusion injury is associated with the p53/TIGAR pathway: Effect of fructose 1,6-diphosphate. PeerJ 2016, 4, e2195. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.G. Testicular biopsy score count—A method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1970, 1, 2–25. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound NQDI-1 is available from the authors. |







| Gene Name | Sham | tIRI | * p-Value | NQDI-1 1 | #p-Value |
|---|---|---|---|---|---|
| Pro-Apoptosis Genes | |||||
| Bax I | 1.0 ± 0.0 | 2.4 ± 0.8 | <0.0001 | 0.8 ± 0.1 | <0.0001 |
| C | 1.0 ± 0.0 | 0.9 ± 0.4 | >0.9999 | 0.8 ± 0.3 | 0.9957 |
| Bid I | 1.0 ± 0.0 | 13 ± 1.9 | <0.0001 | 1.2 ± 0.6 | <0.0001 |
| C | 1.0 ± 0.0 | 1.1 ± 0.1 | >0.9999 | 1.0 ± 0.1 | >0.9999 |
| Bad I | 1.0 ± 0.0 | 7.6 ± 1.5 | <0.0001 | 0.9 ± 0.3 | <0.0001 |
| C | 1.0 ± 0.0 | 0.9 ± 0.1 | >0.9999 | 0.9 ± 0.3 | >0.9999 |
| Anti-Apoptosis Genes | |||||
| Bcl-2 I | 1.0 ± 0.0 | 0.2 ± 0.01 | 0.003 | 1.1 ± 0.2 | 0.001 |
| C | 1.0 ± 0.0 | 1.1 ± 0.5 | >0.9999 | 0.9 ± 0.7 | 0.9996 |
| Birc5 I | 1.0 ± 0.0 | 0.2 ± 0.04 | <0.0001 | 0.9 ± 0.2 | <0.0001 |
| (Survivin) C | 1.0 ± 0.0 | 1.1 ± 0.2 | 0.5772 | 1.0 ± 0.1 | 0.7213 |
| Bax/Bcl-2 I | 1.0 ± 0.0 | 17 ± 1.6 | 0.0012 | 1.3 ± 0.4 | 0.0014 |
| Ratio C | 1.0 ± 0.0 | 0.9 ± 0.1 | >0.9999 | 1.6 ± 0.8 | >0.9999 |
| Gene Name | Sham | tIRI | * p-Value | NQDI-1 1 | #p-Value |
|---|---|---|---|---|---|
| Txn1 IC | 1.0 ± 0.00 | 0.4 ± 0.09 | <0.0001 | 0.0 ± 0.21 | <0.0001 |
| 1.0 ± 0.00 | 0.8 ± 0.15 | 0.1827 | 0.8 ± 0.24 | 0.9927 | |
| Txn2 IC | 1.0 ± 0.00 | 0.2 ± 0.04 | <0.0001 | 0.9 ± 0.15 | <0.0001 |
| 1.0 ± 0.00 | 0.8 ± 0.15 | 0.0973 | 0.8 ± 0.24 | 0.9860 | |
| Txnrd1 IC | 1.0 ± 0.00 | 0.1 ± 0.16 | <0.0001 | 0.9 ± 0.09 | <0.0001 |
| 1.0 ± 0.00 | 0.9 ± 0.08 | 0.0837 | 0.9 ± 0.09 | >0.9999 | |
| Txnrd2 IC | 1.0 ± 0.00 | 0.2 ± 0.07 | <0.0001 | 0.9 ± 0.22 | <0.0001 |
| 1.0 ± 0.00 | 1.0 ± 0.18 | >0.9999 | 0.9 ± 0.22 | 0.9453 | |
| Txnip IC | 1.0 ± 0.00 | 6.3 ± 1.10 | <0.0001 | 1.1 ± 0.41 | <0.0001 |
| 1.0 ± 0.00 | 1.4 ± 0.93 | 0.7973 | 1.3 ± 0.43 | 0.9994 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Kandari, N.; Fadel, F.; Al-Saleh, F.; Khashab, F.; Al-Maghrebi, M. The Thioredoxin System is Regulated by the ASK-1/JNK/p38/Survivin Pathway During Germ Cell Apoptosis. Molecules 2019, 24, 3333. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24183333
Al-Kandari N, Fadel F, Al-Saleh F, Khashab F, Al-Maghrebi M. The Thioredoxin System is Regulated by the ASK-1/JNK/p38/Survivin Pathway During Germ Cell Apoptosis. Molecules. 2019; 24(18):3333. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24183333
Chicago/Turabian StyleAl-Kandari, Nora, Fatemah Fadel, Farah Al-Saleh, Farah Khashab, and May Al-Maghrebi. 2019. "The Thioredoxin System is Regulated by the ASK-1/JNK/p38/Survivin Pathway During Germ Cell Apoptosis" Molecules 24, no. 18: 3333. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24183333