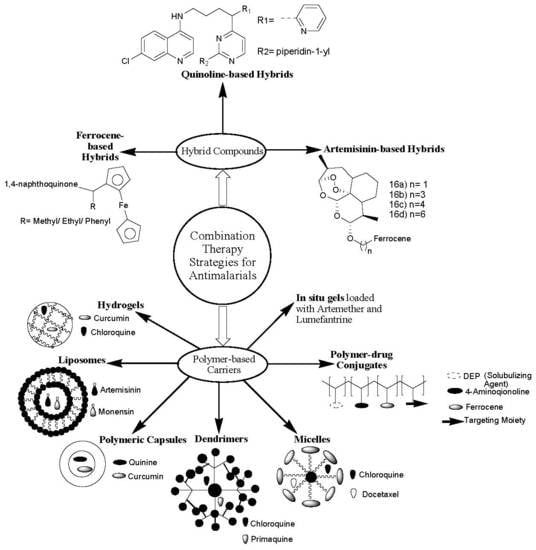
Combination Therapy Strategies for the Treatment of Malaria
Abstract
:1. Introduction
2. Classification of Antimalarial Drugs
3. Combination Therapy Strategies
3.1. Antimalarial Hybrid Compounds
3.1.1. Artemisinin-Based Hybrid Compounds
3.1.2. Nonartemisinin-Based Hybrid Compounds
Quinoline-Based Hybrid Compounds
Ferrocene-Based Hybrid Compounds
3.2. Polymer-Based Carriers for Antimalarial Drug Combination Therapies
3.2.1. Polymer–Drug Conjugates
3.2.2. Micelles and Dendrimers
3.2.3. Hydrogels and in Situ Gels
3.2.4. Nano- and Microcapsules
3.2.5. Polymeric Nanoparticles
3.2.6. Liposomes
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Sultana, M.; Sheikh, N.; Mahumud, R.A.; Jahir, T.; Islam, Z.; Sarker, A.R. Prevalence and associated determinants of malaria parasites among Kenyan children. Trop. Med. Health 2017, 45, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkholtz, L.-M.; Bornman, R.; Focke, W.; Mutero, C.; de Jager, C. Sustainable malaria control: Transdisciplinary approaches for translational applications. Malar. J. 2012, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Antinori, S.; Galimberti, L.; Milazzo, L.; Corbellino, M. Biology of Human Malaria Plasmodia Including Plasmodium Knowlesi. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012013. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 12 October 2018).
- Farooq, U.; Mahajan, R.C. Drug resistance in malaria. J. Vector Borne Dis. 2004, 41, 45–53. [Google Scholar]
- Takala-Harrison, S.; Laufer, M.K. Antimalarial drug resistance in Africa: Key lessons for the future. Ann. N. Y. Acad. Sci. 2015, 1342, 62–67. [Google Scholar] [CrossRef]
- Starzengruber, P.; Fuehrer, H.-P.; Swoboda, P.; Ganesh, D.; Haque, R.; Akhan, W. Mirincamycin, an old candidate for malaria combination treatment and prophylaxis in the 21st century: In vitro interaction profiles with potential partner drugs in continuous culture and field isolates. Malar. J. 2014, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Phyo, A.P. Drugs in Development for Malaria. Drugs 2018, 78, 861–879. [Google Scholar] [CrossRef] [Green Version]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022. [Google Scholar] [CrossRef]
- Yan, G.; Li, A.; Zhang, A.; Sun, Y.; Liu, J. Polymer-Based Nanocarriers for Co-Delivery and Combination of Diverse Therapies against Cancers. Nanomaterials 2018, 8, 85. [Google Scholar] [CrossRef]
- Whegang, S.Y.; Tahar, R.; Foumane, V.N.; Soula, G.; Gwét, H.; Thalabard, J.; Basco, L.K. Efficacy of non-artemisinin- and artemisinin-based combination therapies for uncomplicated falciparum malaria in Cameroon. Malar. J. 2010, 9, 56. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.N.H.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Elvira, C.; Gallardo, A.; San Roman, J.; Cifuentes, A. Covalent polymer–drug conjugates. Molecules 2005, 10, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Marturano, V.; Cerruti, P.; Giamberini, M.; Tylkowski, B.; Ambrogi, V. Light-Responsive Polymer Micro- and Nano-Capsules. Polymers 2017, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, H.B.; Bakliwal, S.R.; Pawar, S.P. In-Situ gel: New trends in Controlled and Sustained Drug Delivery System. Int. J. PharmTech Res. 2010, 2, 1398–1408. [Google Scholar]
- Xu, W.; Ling, P.; Zhang, T. Polymeric Micelles, a Promising Drug Delivery System to Enhance Bioavailability of Poorly Water-Soluble Drugs. J. Drug Deliv. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mhlwatika, Z.; Aderibigbe, B.A. Polymeric Nanocarriers for the Delivery of Antimalarials. Molecules 2018, 23, 2527. [Google Scholar] [CrossRef] [PubMed]
- Sevene, E.; González, R.; Menéndez, C. Expert Opinion on Pharmacotherapy Current knowledge and challenges of antimalarial drugs for treatment and prevention in pregnancy. Expert Opin. Pharmacother. 2010, 11, 1277–1293. [Google Scholar] [CrossRef]
- Dow, G.S.; Ohrt, C. Clinical development of new prophylactic antimalarial drugs after the 5th Amendment to the Declaration of Helsinki. Ther. Clin. Risk Manag. 2008, 4, 803–819. [Google Scholar] [CrossRef]
- World Health Organization. The Use of Antimalarial Drugs Report of an Informal Consultation; World Health Organization: Geneva, Switzerland, 2000; pp. 1–144. [Google Scholar]
- South African Guidelines for the Prevention of Malaria. 2017. Available online: http://www.nicd.ac.za/wp-content/uploads/2017/09/Guidelines-South-African-Guidelines-for-the-Prevention-of-Malaria-2017-final.pdf (accessed on 3 June 2019).
- Nqoro, X.; Naki, T.; Aderibigbe, B.A. Quinoline-Based Hybrid Compounds with Antimalarial Activity. Molecules 2017, 22, 2268. [Google Scholar] [CrossRef]
- Smit, F.J.; van Biljon, R.A.; Birkholtz, L.; Da, D.D.N. Synthesis and in vitro biological evaluation of dihydroartemisinyl-chalcone esters. Eur. J. Med. Chem. 2015, 90, 33–44. [Google Scholar] [CrossRef]
- Lange, C.D.; Coertzen, D.; Smit, F.J.; Wentzel, J.F.; Ning, H.; Birkholtz, L.; Haynes, R.K.; Da, D.D.N. Synthesis, in vitro antimalarial activities and cytotoxicities of amino-artemisinin-ferrocene derivatives. Bioorg. Med. Chem. Lett. 2018, 28, 289–292. [Google Scholar] [CrossRef]
- Lange, C.D.; Coertzen, D.; Smit, F.J.; Wentzel, J.F.; Ning, H.; Birkholtz, L.; Haynes, R.K.; Da, D.D.N. Synthesis, antimalarial activities and cytotoxicities of amino-artemisinin-1, 2-disubstituted ferrocene hybrids. Bioorg. Med. Chem. Lett. 2018, 28, 3161–3163. [Google Scholar] [CrossRef] [PubMed]
- Çapc, A.; Reiter, C.; Seo, E.; Gruber, L.; Hahn, F.; Leidenberger, M.; Klein, V.; Hampel, F.; Friedrich, O.; Marschall, M.; et al. Access to new highly potent antileukemia, antiviral and antimalarial agents via hybridization of natural products (homo) egonol, thymoquinone and artemisinin. Bioorg. Med. Chem. 2018, 26, 3610–3618. [Google Scholar]
- Wang, N.; Wicht, K.J.; Shaban, E.; Ngoc, T.A.; Wang, M.-Q.; Hayashi, I.; Hossain, I.; Takemasa, Y.; Kaiser, M.; El, I.; et al. Synthesis and evaluation of artesunate–indoloquinoline hybrids as antimalarial drug candidates. Medchemcomm 2014, 5, 927–931. [Google Scholar] [CrossRef]
- Walsh, J.J.; Coughlan, D.; Heneghan, N.; Bell, A. Novel artemisinin–quinine hybrid with potent antimalarial activity. Bioorg. Med. Chem. Lett. 2007, 17, 3599–3602. [Google Scholar] [CrossRef]
- Joubert, J.P.; Smit, F.J.; Smith, P.J.; Da, D.D.N. Synthesis and in vitro biological evaluation of aminoacridines and artemisinin—Acridine hybrids. Eur. J. Pharm. Sci. 2014, 56, 16–27. [Google Scholar] [CrossRef]
- Raj, H.; Pratap, U.; Yadav, P.S.; Kumar, V.; Gahtori, P.; Das, A.; Chetia, D.; Prakash, A.; Mahanta, J. Synthesis, characterization and antimalarial activity of hybrid 4-aminoquinoline-1, 3, 5-triazine derivatives. Arab. J. Chem. 2016, 9, s625–s631. [Google Scholar]
- Sahu, S.; Kumar, S.; Kalita, J.; Dutta, M.; Raj, H. Design, synthesis and antimalarial screening of some hybrid 4-aminoquinoline-triazine derivatives against pf-DHFR-TS. Exp. Parasitol. 2016, 163, 38–45. [Google Scholar] [CrossRef]
- Raj, H.; Pratap, U.; Thakur, A.; Kumar, S.; Gogoi, K.; Prakash, A.; Singh, R.K. Synthesis, antimalarial activity and molecular docking of hybrid. Exp. Parasitol. 2015, 157, 59–67. [Google Scholar]
- Maurya, S.S.; Bahuguna, A.; Khan, S.I.; Kumar, D.; Kholiya, R.; Rawat, D.S. N-Substituted aminoquinoline-pyrimidine hybrids: Synthesis, in vitro antimalarial activity evaluation and docking studies. Eur. J. Med. Chem. 2019, 162, 277–289. [Google Scholar] [CrossRef]
- Shyam, S.; Khan, S.I.; Bahuguna, A.; Kumar, D.; Rawat, D.S. Synthesis, antimalarial activity, heme binding and docking studies of N-substituted 4-aminoquinoline-pyrimidine molecular hybrids. Eur. J. Med. Chem. 2017, 129, 175–185. [Google Scholar]
- Kaur, H.; Balzarini, J.; De Kock, C.; Smith, P.J.; Chibale, K.; Singh, K. Synthesis, antiplasmodial activity and mechanistic studies of pyrimidine-5-carbonitrile and quinoline hybrids. Eur. J. Med. Chem. 2015, 101, 52–62. [Google Scholar] [CrossRef]
- Kholiya, R.; Khan, S.I.; Bahuguna, A.; Tripathi, M. N-Piperonyl substitution on aminoquinoline-pyrimidine hybrids: Effect on the antiplasmodial potency. Eur. J. Med. Chem. 2017, 131, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Raichurkar, A.V.; Rahman, F.; Khan, N.; Iyer, P.S. Synthesis and in vitro evaluation of novel 8-aminoquinoline—Pyrazolopyrimidine hybrids as potent antimalarial agents. Bioorg. Med. Chem. Lett. 2015, 25, 1100–1103. [Google Scholar]
- Reddy, P.L.; Khan, S.I.; Ponnan, P.; Tripathi, M.; Rawat, D.S. Design, synthesis and evaluation of 4-aminoquinoline-purine hybrids as potential antiplasmodial agents. Eur. J. Med. Chem. 2017, 126, 675–686. [Google Scholar] [CrossRef] [Green Version]
- Capela, R.; Joana, M.; Miranda, D.; Machado, M.; Capela, R.; Magalh, J.; Rosenthal, P.J.; Frade, R.; Perry, M.J.; Moreira, R.; et al. Endoperoxide-8-aminoquinoline hybrids as dual-stage antimalarial agents with enhanced metabolic stability. Eur. J. Med. Chem. 2018, 149, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Reis, R.; Marcio, J.; Cecheto, B.; Campos, C.; Salomé, L.; Souza, A.D.; Valério, F.; Mafra, R.; Dias, D.P.; Otávio, P.; et al. New quinoline derivatives demonstrate a promising antimalarial activity against Plasmodium falciparum in vitro and Plasmodium berghei in vivo. Bioorg. Med. Chem. Lett. 2015, 25, 2308–2313. [Google Scholar]
- Pinheiro, L.C.S.; Boechat, N.; Lourdes, M.D.; Ferreira, G.; Júnior, C.C.S.; Jesus, A.M.L.; Leite, M.M.M.; Souza, N.B.; Krettli, A.U. Anti-Plasmodium falciparum activity of quinoline–sulfonamide hybrids. Bioorg. Med. Chem. 2015, 23, 5979–5984. [Google Scholar] [CrossRef]
- Barteselli, A.; Parapini, S.; Basilico, N.; Mommo, D.; Sparatore, A. Synthesis and evaluation of the antiplasmodial activity of novel indeno [2,1-c] quinoline derivatives. Bioorg. Med. Chem. 2014, 22, 5757–5765. [Google Scholar] [CrossRef]
- Silva, R.M.R.J.; Gandi, M.O.; Mendonça, J.S.; Carvalho, A.S.; Penna, J.; Aguiar, A.C.C.; Krettli, A.U.; Boechat, N. New hybrid trifluoromethylquinolines as antiplasmodium agents. Bioorg. Med. Chem. 2019, 27, 1002–1008. [Google Scholar] [CrossRef]
- Bonilla-ramirez, L.; Rios, A.; Quiliano, M.; François, J.; Ramirez-calderon, G.; Corcuera, L.; Bordessoulles, M.; Vettorazzi, A.; Adela, L.; Aldana, I.; et al. Novel antimalarial chloroquine- and primaquine-quinoxaline 1,4-di-N-oxide hybrids: Design, synthesis, Plasmodium life cycle stage profile and preliminary toxicity studies. Eur. J. Med. Chem. 2018, 158, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Legac, J.; Rosenthal, P.J.; Kumar, V. Substituted 1,3-dioxoisoindoline-4-aminoquinolines coupled via amide linkers: Synthesis, antiplasmodial and cytotoxic evaluation. Bioorg. Chem. 2019, 88, 102912. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.C.; Martins, W.A.; Silva, T.P.; Kaiser, C.R.; Bastos, M.M.; Pinheiro, L.C.S.; Krettli, A.U.; Boechat, N. New pentasubstituted pyrrole hybrid atorvastatin—Quinoline derivatives with antiplasmodial activity. Bioorg. Med. Chem. Lett. 2016, 26, 1881–1884. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Pradines, B.; Madamet, M.; Kumar, V. Lactam conjugates: Synthesis and antimalarial evaluation. Eur. J. Med. Chem. 2014, 86, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Saini, A.; Gut, J.; Rosenthal, P.J.; Kumar, V. Synthesis and in vitro antiplasmodial evaluation of 7-chloroquinoline e chalcone and 7-chloroquinoline e ferrocenylchalcone conjugates. Eur. J. Med. Chem. 2015, 95, 230–239. [Google Scholar] [CrossRef] [PubMed]
- García-barrantes, P.M.; Lamoureux, G.V.; Pérez, A.L.; García-sánchez, R.N.; Martínez, A.R.; San, A. European Journal of Medicinal Chemistry Synthesis and biological evaluation of novel ferrocene e naphthoquinones as antiplasmodial agents. Eur. J. Med. Chem. 2013, 70, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Kondratskyi, A.; Kondratska, K.; Abeele, F.V.; Gordienko, D.; Dubois, C.; Toillon, R.; Slomianny, C.; Lemière, S.; Delcourt, P.; Dewailly, E.; et al. Ferroquine, the next generation antimalarial drug, has antitumor activity. Sci. Rep. 2017, 7, 15896. [Google Scholar] [CrossRef] [PubMed]
- Biot, C.; Taramelli, D.; Forfar-Bares, I.; Maciejewski, L.A.; Boyce, M.; Guy, N.; Brocard, J.S.; Basilico, N.; Olliaro, P.; Egan, T.J. Insights into the Mechanism of Action of Ferroquine. Relationship between Physicochemical Properties and Antiplasmodial Activity. Mol. Pharm. 2005, 2, 185–193. [Google Scholar] [CrossRef]
- Biot, C.; Chavain, N.; Dubar, F.; Pradines, B.; Trivelli, X.; Brocar, J.; Forfar, I.; Dive, D. Structure–activity relationships of 4-N-substituted ferroquine analogues: Time to re-evaluate the mechanism of action of ferroquine. J. Organomet. Chem. 2009, 694, 45–854. [Google Scholar] [CrossRef]
- Chavain, N.; Vezin, H.; Dive, D.; Touati, N.; Paul, J.-F.; Buisine, E.; Biot, C. Investigation of the Redox Behavior of Ferroquine, a New Antimalarial. Mol. Pharm. 2008, 5, 710–716. [Google Scholar] [CrossRef]
- Chopra, R.; De Kock, C.; Smith, P.; Chibale, K.; Singh, K. Ferrocene-pyrimidine conjugates: Synthesis, electrochemistry, physicochemical properties and antiplasmodial activities. Eur. J. Med. Chem. 2015, 100, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ringsdorf, H. Structure and properties of pharmacologically active polymers. J. Polym. Sci. Polym. Symp. 1975, 5, 135–153. [Google Scholar] [CrossRef]
- Larson, N.; Ghandehari, H. Polymeric conjugates for drug delivery. Chem. Mater. 2012, 13, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Marasini, N.; Haque, S.; Kaminskas, L.M. Polymer–drug conjugates as inhalable drug delivery systems: A review. Curr. Opin. Colloid Interface Sci. 2017, 31, 18–29. [Google Scholar] [CrossRef]
- Dragojevic, S.; Ryu, J.S.; Raucher, D. Polymer-Based Prodrugs: Improving Tumor Targeting and the Solubility of Small Molecule Drugs in Cancer Therapy. Molecules 2015, 20, 21750–21769. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Yadav, T.; Sharma, S.; Nayak, A.; Kumari, A. Polymers in Drug Delivery. J. Biosci. Med. 2016, 4, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Singh, R.K.; Sharma, R.; Murthy, R.S.R.; Bhardwaj, T.R. Design, synthesis and evaluation of antimalarial potential of polyphosphazene linked combination therapy of primaquine and dihydroartemisinin. Eur. J. Pharm. Sci. 2015, 66, 123–137. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Ray, S.S. Preparation, characterization and in vitro release kinetics of polyaspartamide-based conjugates containing antimalarial and anticancer agents for combination therapy. J. Drug Deliv. Sci. Technol. 2016, 36, 34–45. [Google Scholar] [CrossRef]
- Urbán, P.; Valle-delgado, J.J.; Mauro, N.; Marques, J.; Manfredi, A.; Rottmann, M.; Ranucci, E.; Ferruti, P.; Fernàndez-busquets, X. Use of poly (amidoamine) drug conjugates for the delivery of antimalarials to Plasmodium. J. Control. Release 2014, 177, 84–95. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Mhlwatika, Z.; Nwamadi, M.; Balogun, M.O.; Matshe, W.M.R. Synthesis, characterization and in vitro analysis of polymer-based conjugates containing dihydrofolate reductase inhibitors. J. Drug Deliv. Sci. Technol. 2019, 50, 388–401. [Google Scholar] [CrossRef]
- Starov, V.; Zhdanov, V.; Kovalchuk, N. Kinetic models of micelles formation. J. Colloids Surf. 2010, 354, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Danafar, H.; Rostamizadeh, K.; Davaran, S.; Hamidi, M. Drug-conjugated PLA–PEG–PLA copolymers: A novel approach for controlled delivery of hydrophilic drugs by micelle formation. Pharm. Dev. Technol. 2017, 22, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Husseini, G.A.; Pitt, W.G. Micelles and Nanoparticles for Ultrasonic Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kanamoto, T.; Nakashima, H.; Yoshida, T. Synthesis of a new amphiphilic glycodendrimer with antiviral functionality. Carbohydr. Polym. 2012, 90, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.V.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Zhang, Z.; Shi, J.; Wang, F.; Luan, Y. Co-delivery of docetaxel and chloroquine via PEO-PPO-PCL/TPGS micelles for overcoming multidrug resistance. Int. J. Pharm. 2015, 495, 932–939. [Google Scholar] [CrossRef]
- Movellan, J.; Urban, P.; Moles, E.; Fuente, J.M.; Sierra, T.; Serrano, J.L.; Fernandez-busquetts, X. Amphiphilic dendritic derivatives as nanocarriers for the targeted delivery of antimalarial drugs. Biomat. 2014, 13, 7940–7950. [Google Scholar] [CrossRef]
- Kopecek, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [Green Version]
- Sarada, K.; Firoz, S.; Padmini, K. In-Situ Gelling System: A Review. Int. J. Curr. Pharm. Rev. Res. 2014, 5, 76–90. [Google Scholar]
- Aderibigbe, B.A.; Sadiku, E.; Jayaramudu, J.; Ray, S.S. Controlled Dual Release Study of Curcumin and a 4-Aminoquinoline Analog from Gum Acacia Containing Hydrogels. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Mhlwatika, Z. Dual release kinetics of antimalarials from soy protein isolate-carbopol-polyacrylamide based hydrogels. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Dawre, S.; Pathak, S.; Sharma, S.; Devarajan, P.V. Enhanced antimalalarial activity of a prolonged release in situ gel of arteether–lumefantrine in a murine model. Eur. J. Pharm. Biopharm. 2018, 123, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Kothamasu, P.; Kanumur, H.; Ravur, N.; Maddu, C.; Parasuramrajam, R.; Thangavel, S. Nanocapsules: The Weapons for Novel Drug Delivery Systems. BioImpacts 2012, 2, 71–81. [Google Scholar] [PubMed]
- Ariga, K.; Lvov, Y.M.; Kawakami, K.; Ji, Q.; Hill, J.P. Layer-by-layer self-assembled shells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Hemant, K.; Ram, M.; Shivakumar, H. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 67–77. [Google Scholar]
- Velasques, K.; Ramos, T.; Helena, A.; Dal, D.C.; Elizabete, F.; Teixeira, G.; Luisa, A.; Pilla, F.D.; Ricardo, A.; Silva, D.; et al. Co-nanoencapsulation of antimalarial drugs increases their in vitro efficacy against Plasmodium falciparum and decreases their toxicity to Caenorhabditis elegans. Eur. J. Pharm. Sci. 2018, 118, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, A.K.; Amareshwar, P.; Chakravarty, P. Different Techniques Used for the Preparation of Nanoparticles Using Natural Polymers and Their Application. Int. J. Pharm. Pharm. Sci. 2011, 3, 45–50. [Google Scholar]
- Wu, Y.; Yang, W.; Wang, C.; Hu, J.; Fu, S. Chitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinate. Int. J. Pharm. Sci. Res. 2005, 295, 235–245. [Google Scholar] [CrossRef]
- Dennis, E.; Peoples, V.A.; Johnson, F.; Bibbs, R.K.; Topps, D.; Bopda-Waffo, A.; Coats, M.T. Utilizing Nanotechnology to Combat Malaria. J. Infect. Dis. Ther. 2015, 3, 1–6. [Google Scholar]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Jawahar, N.; Baruah, U.K.; Singh, V. Co-delivery of chloroquine phosphate and azithromycin nanoparticles to overcome drug resistance in malaria through intracellular targeting. J. Pharm. Sci. Res. 2019, 11, 33–40. [Google Scholar]
- Anand, R.; Manoli, F.; Manet, L.; Daoud-Mahammed, S.; Agostoni, V.; Gref, R.; Monti, S. artemisinin: A spectroscopic and photophysical study. Photochem. Photobiol. Sci. 2012, 11, 1285–1292. [Google Scholar] [CrossRef]
- Oyeyemi, O.; Morenkeji, O.; Afolayan, F.; Dauda, K.; Busari, Z.; Meena, J.; Panda, A. Curcumin-Artesunate Based Polymeric Nanoparticle; Antiplasmodial and Toxicological Evaluation in Murine Model. Front. Pharmacol. 2018, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Labhasetwar, V. Nanotech approaches to drug delivery and imaging. Drug Descov. Today 2003, 8, 1112–1120. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Nas, F.S.; Yahaya, A.; Ali, M. Application of Liposomes Nanoparticles in the Treatment of Malaria: A Mini Review. J. Biotechnol. Bioresearch 2018, 1, 1–5. [Google Scholar]
- Wagner, A.; Vorauer-uhl, K. Liposome Technology for Industrial Purposes. J. Drug Deliv. 2001, 2011, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shehata, T.; Ogawara, K.; Higaki, K.; Kimura, T. Prolongation of residence time of liposome by surface-modification with mixture of hydrophilic polymers. Int. J. Pharm. 2008, 359, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.J.W.; Semple, S.C.; Klimuk, S.K.; Ansell, S.; Maurer, N.; Cullis, P.R. Characterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelin. Biochim. Biophys. Acta 2007, 1768, 1121–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.; Tagami, T.; Ozeki, T. Effective-Loading of Platinum—Chloroquine into PEGylated Neutral and Cationic Liposomes as a Drug Delivery System for Resistant Malaria Parasites. Biol. Pharm. Bull. 2017, 40, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Aditya, N.P.; Chimote, G.; Gunalan, K.; Banerjee, R.; Patankar, S.; Madhusudhan, B. Curcuminoids-loaded liposomes in combination with arteether protects against Plasmodium berghei infection in mice. Exp. Parasitol. 2012, 131, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, V.; Rohra, S.; Raza, M.; Hasan, M.; Dutt, S.; Ghosh, C. Stearylamine Liposomal Delivery of Monensin in Combination with Free Artemisinin Eliminates Blood Stages of Plasmodium falciparum in Culture and P berghei Infection in Murine Malaria. Antimicrob. Agents Chemother. 2016, 60, 1304–1318. [Google Scholar] [CrossRef] [PubMed]






















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alven, S.; Aderibigbe, B. Combination Therapy Strategies for the Treatment of Malaria. Molecules 2019, 24, 3601. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24193601
Alven S, Aderibigbe B. Combination Therapy Strategies for the Treatment of Malaria. Molecules. 2019; 24(19):3601. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24193601
Chicago/Turabian StyleAlven, Sibusiso, and Blessing Aderibigbe. 2019. "Combination Therapy Strategies for the Treatment of Malaria" Molecules 24, no. 19: 3601. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24193601