MicroRNA-29a Attenuates Diabetic Glomerular Injury through Modulating Cannabinoid Receptor 1 Signaling
Abstract
:1. Introduction
2. Results
2.1. MiR-29a Overexpression Significantly Reduces Diabetes-Induced Renal Hypertrophy
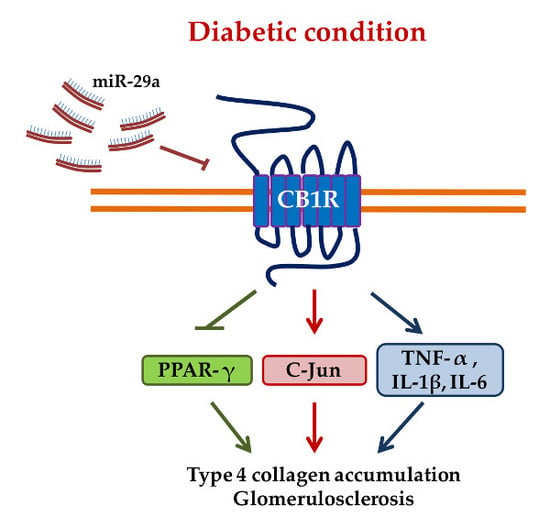
2.2. MiR-29a Modulates CB1R Expression and Ameliorates Renal Fibrotic Injuries
2.3. MiR-29a Attenuates CB1R but Restores Peroxisome Proliferator-Activated Receptor-γ (PPAR-γ) Expressions in Diabetic Animals
3. Discussion
4. Materials and Methods
4.1. In Vitro High-Glucose-Treated Mesangial Cell Cultures
4.2. Transfection of MicroRNA-29a (miR-29a) Precursor
4.3. Protein Extraction and Western Blotting Analysis
4.4. MiR-29a Transgenic Mice
4.5. Streptozotocin (STZ)-Induced Diabetic Animal Models
4.6. Blood Biochemistry and Measurement of Kidney Weight
4.7. Preparation and Laser Capture Microdissection (LCM) of Kidney Tissues
4.8. Quantitative Reverse Transcription-PCR (qRT-PCR)
4.9. Immunohistochemistry
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Kanasaki, K.; Taduri, G.; Koya, D. Diabetic nephropathy: The role of inflammation in fibroblast activation and kidney fibrosis. Front. Endocrinol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Umanath, K.; Lewis, J.B. Update on Diabetic Nephropathy: Core Curriculum 2018. Am. J. Kidney Dis. 2018, 71, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Busquets-Garcia, A.; Gomis-Gonzalez, M.; Srivastava, R.K.; Cutando, L.; Ortega-Alvaro, A.; Ruehle, S.; Remmers, F.; Bindila, L.; Bellocchio, L.; Marsicano, G.; et al. Peripheral and central CB1 cannabinoid receptors control stress-induced impairment of memory consolidation. Proc. Natl. Acad. Sci. USA 2016, 113, 9904–9909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.Y.; Alexa, K.; Cortes, M.; Schatzman-Bone, S.; Kim, A.J.; Mukhopadhyay, B.; Cinar, R.; Kunos, G.; North, T.E.; Goessling, W. Cannabinoid receptor signaling regulates liver development and metabolism. Development 2016, 143, 609–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurier, R.B.; Burstein, S.H. Cannabinoids, inflammation, and fibrosis. FASEB J. 2016, 30, 3682–3689. [Google Scholar] [CrossRef]
- Cinar, R.; Gochuico, B.R.; Iyer, M.R.; Jourdan, T.; Yokoyama, T.; Park, J.K.; Coffey, N.J.; Pri-Chen, H.; Szanda, G.; Liu, Z.; et al. Cannabinoid CB1 receptor overactivity contributes to the pathogenesis of idiopathic pulmonary fibrosis. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Michalski, C.W.; Maier, M.; Erkan, M.; Sauliunaite, D.; Bergmann, F.; Pacher, P.; Batkai, S.; Giese, N.A.; Giese, T.; et al. Cannabinoids Reduce Markers of Inflammation and Fibrosis in Pancreatic Stellate Cells. PLoS ONE 2008, 3, e1701. [Google Scholar] [CrossRef]
- Servettaz, A.; Kavian, N.; Nicco, C.; Deveaux, V.; Chereau, C.; Wang, A.; Zimmer, A.; Lotersztajn, S.; Weill, B.; Batteux, F. Targeting the cannabinoid pathway limits the development of fibrosis and autoimmunity in a mouse model of systemic sclerosis. Am. J. Pathol. 2010, 177, 187–196. [Google Scholar] [CrossRef]
- Teixeira-Clerc, F.; Julien, B.; Grenard, P.; Tran Van Nhieu, J.; Deveaux, V.; Li, L.; Serriere-Lanneau, V.; Ledent, C.; Mallat, A.; Lotersztajn, S. CB1 cannabinoid receptor antagonism: A new strategy for the treatment of liver fibrosis. Nat. Med. 2006, 12, 671–676. [Google Scholar] [CrossRef]
- Lecru, L.; Desterke, C.; Grassin-Delyle, S.; Chatziantoniou, C.; Vandermeersch, S.; Devocelle, A.; Vernochet, A.; Ivanovski, N.; Ledent, C.; Ferlicot, S.; et al. Cannabinoid receptor 1 is a major mediator of renal fibrosis. Kidney Int. 2015, 88, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Cao, X.; Zhang, K.; Li, Y.; Zheng, Q.Y.; Li, G.Q.; He, Q.H.; Li, S.J.; Xu, G.L.; Zhang, K.Q. Celastrol alleviates renal fibrosis by upregulating cannabinoid receptor 2 expression. Cell Death Dis. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, S.; Yang, P.; Tian, Y.; Feng, Z.; Xie, X.-Q.; Liu, Y. Targeted inhibition of the type 2 cannabinoid receptor is a novel approach to reduce renal fibrosis. Kidney Int. 2018, 94, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Bruno, G.; Mastrocola, R.; Bellini, S.; Gruden, G. The role of cannabinoid signaling in acute and chronic kidney diseases. Kidney Int. 2018, 94, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Lei, C.C.; Shih, Y.H.; Ho, C.; Lin, C.L. Induction of proteinuria by cannabinoid receptors 1 signaling activation in CB1 transgenic mice. Am. J. Med. Sci. 2015, 349, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Grimaldi, S.; Franco, I.; Bellini, S.; Gambino, R.; Pinach, S.; Corbelli, A.; Bruno, G.; Rastaldi, M.P.; Aveta, T.; et al. Deficiency of cannabinoid receptor of type 2 worsens renal functional and structural abnormalities in streptozotocin-induced diabetic mice. Kidney Int. 2014, 86, 979–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Mi, Q.-S.; Dong, Z. The regulation and function of micrornas in kidney diseases. IUBMB Life 2013, 65, 602–614. [Google Scholar] [CrossRef] [Green Version]
- Ichii, O.; Horino, T. MicroRNAs associated with the development of kidney diseases in humans and animals. J. Toxicol. Pathol. 2018, 31, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, K.; Lanting, L.L.; Jia, Y.; Yadav, S.; Reddy, M.A.; Magilnick, N.; Boldin, M.; Natarajan, R. Anti-Inflammatory Role of MicroRNA-146a in the Pathogenesis of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2015, 27, 2277–2288. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.L.; Lee, P.H.; Hsu, Y.C.; Lei, C.C.; Ko, J.Y.; Chuang, P.C.; Huang, Y.T.; Wang, S.Y.; Wu, S.L.; Chen, Y.S.; et al. MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction. J. Am. Soc. Nephrol. 2014, 25, 1698–1709. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Chang, P.J.; Ho, C.; Huang, Y.T.; Shih, Y.H.; Wang, C.J.; Lin, C.L. Protective effects of miR-29a on diabetic glomerular dysfunction by modulation of DKK1/Wnt/beta-catenin signaling. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, A.J.; Liu, Y.; Fang, Y.; Ding, X.; Liang, M. The miR-29 family: Genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol. Genomics 2012, 44, 237–244. [Google Scholar] [CrossRef] [PubMed]
- De Borst, M.H.; Prakash, J.; Melenhorst, W.B.; van den Heuvel, M.C.; Kok, R.J.; Navis, G.; van Goor, H. Glomerular and tubular induction of the transcription factor c-Jun in human renal disease. J. Pathol. 2007, 213, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.G.; Feld, S.; Striker, L.; Striker, G.; LaPage, J.; Esposito, C.; Aboulhosn, J.; Barba, L.; Cha, D.R.; Nast, C.C. Glomerular type IV collagen in patients with diabetic nephropathy with and without additional glomerular disease. Kidney Int. 2000, 57, 2084–2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umezono, T.; Toyoda, M.; Kato, M.; Miyauchi, M.; Kimura, M.; Maruyama, M.; Honma, M.; Yagame, M.; Suzuki, D. Glomerular expression of CTGF, TGF-beta 1 and type IV collagen in diabetic nephropathy. J. Nephrol. 2006, 19, 751–757. [Google Scholar] [PubMed]
- Balakumar, P.; Arora, M.K.; Singh, M. Emerging role of PPAR ligands in the management of diabetic nephropathy. Pharmacol. Res. 2009, 60, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, Y.; Guan, Y. PPARgamma as a therapeutic target in diabetic nephropathy and other renal diseases. Curr. Opin. Nephrol. Hypertens. 2012, 21, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Ma, L.M.; Huang, M.B.; Zhou, H.; Huang, H.L.; Shao, P.; Chen, Y.Q.; Qu, L.H. High glucose down-regulates miR-29a to increase collagen IV production in HK-2 cells. FEBS Lett. 2010, 584, 811–816. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Komers, R.; Carew, R.; Winbanks, C.E.; Xu, B.; Herman-Edelstein, M.; Koh, P.; Thomas, M.; Jandeleit-Dahm, K.; Gregorevic, P.; et al. Suppression of microRNA-29 expression by TGF-beta1 promotes collagen expression and renal fibrosis. J. Am. Soc. Nephrol. 2012, 23, 252–265. [Google Scholar] [CrossRef]
- Guo, J.; Li, J.; Zhao, J.; Yang, S.; Wang, L.; Cheng, G.; Liu, D.; Xiao, J.; Liu, Z.; Zhao, Z. MiRNA-29c regulates the expression of inflammatory cytokines in diabetic nephropathy by targeting tristetraprolin. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Long, J.; Wang, Y.; Wang, W.; Chang, B.H.; Danesh, F.R. MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J. Biol. Chem. 2011, 286, 11837–11848. [Google Scholar] [CrossRef]
- Lin, C.L.; Hsu, Y.C.; Lee, P.H.; Lei, C.C.; Wang, J.Y.; Huang, Y.T.; Wang, S.Y.; Wang, F.S. Cannabinoid receptor 1 disturbance of PPARgamma2 augments hyperglycemia induction of mesangial inflammation and fibrosis in renal glomeruli. J. Mol. Med. 2014, 92, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, K.; Shi, S.; Kanasaki, M.; He, J.; Nagai, T.; Nakamura, Y.; Ishigaki, Y.; Kitada, M.; Srivastava, S.P.; Koya, D. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes 2014, 63, 2120–2131. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Stafylas, P.C.; Georgianos, P.I.; Saratzis, A.N.; Lasaridis, A.N. Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: A meta-analysis. Am. J. Kidney Dis. 2010, 55, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Portius, D.; Sobolewski, C.; Foti, M. MicroRNAs-Dependent Regulation of PPARs in Metabolic Diseases and Cancers. PPAR Res. 2017, 2017, 1–19. [Google Scholar] [CrossRef]
- Hou, X.; Tian, J.; Geng, J.; Li, X.; Tang, X.; Zhang, J.; Bai, X. MicroRNA-27a promotes renal tubulointerstitial fibrosis via suppressing PPARgamma pathway in diabetic nephropathy. Oncotarget 2016, 7, 47760–47776. [Google Scholar] [CrossRef]
- Kurtz, C.L.; Fannin, E.E.; Toth, C.L.; Pearson, D.S.; Vickers, K.C.; Sethupathy, P. Inhibition of miR-29 has a significant lipid-lowering benefit through suppression of lipogenic programs in liver. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- D’Addario, C.; Di Francesco, A.; Pucci, M.; Finazzi Agrò, A.; Maccarrone, M. Epigenetic mechanisms and endocannabinoid signalling. FEBS J. 2013, 280, 1905–1917. [Google Scholar] [CrossRef]
- Mohnle, P.; Schutz, S.V.; Schmidt, M.; Hinske, C.; Hubner, M.; Heyn, J.; Beiras-Fernandez, A.; Kreth, S. MicroRNA-665 is involved in the regulation of the expression of the cardioprotective cannabinoid receptor CB2 in patients with severe heart failure. Biochem. Biophys. Res. Commun. 2014, 451, 516–521. [Google Scholar] [CrossRef]
- Tang, Y.; Bao, J.S.; Su, J.H.; Huang, W. MicroRNA-139 modulates Alzheimer’s-associated pathogenesis in SAMP8 mice by targeting cannabinoid receptor type 2. Genet. Mol. Res. 2017, 16. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Turu, G.; Hunyady, L. Signal transduction of the CB1 cannabinoid receptor. J. Mol. Endocrinol. 2010, 44, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Bosier, B.; Lambert, D.M.; Hermans, E. Reciprocal influences of CB1cannabinoid receptor agonists on ERK and JNK signalling in N1E-115 cells. FEBS Lett. 2008, 582, 3861–3867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idris, A.I.; van’t Hof, R.J.; Greig, I.R.; Ridge, S.A.; Baker, D.; Ross, R.A.; Ralston, S.H. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat. Med. 2005, 11, 774–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.S.; Wang, C.J.; Chen, Y.J.; Chang, P.R.; Huang, Y.T.; Sun, Y.C.; Huang, H.C.; Yang, Y.J.; Yang, K.D. Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1alpha and VEGF-A expression in shock wave-stimulated osteoblasts. J. Biol. Chem. 2004, 279, 10331–10337. [Google Scholar] [CrossRef] [PubMed]
- Kohda, Y.; Murakami, H.; Moe, O.W.; Star, R.A. Analysis of segmental renal gene expression by laser capture microdissection. Kidney Int. 2000, 57, 321–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.L.; Wang, J.Y.; Ko, J.Y.; Huang, Y.T.; Kuo, Y.H.; Wang, F.S. Dickkopf-1 promotes hyperglycemia-induced accumulation of mesangial matrix and renal dysfunction. J. Am. Soc. Nephrol. 2010, 21, 124–135. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the corresponding authors upon requests. |





| WT NC | WT DM | Tg NC | Tg DM | |
|---|---|---|---|---|
| Blood glucose (mg/dL) | 140 ± 11.1 | 439.4 ± 3.6 * | 139 ± 10.1 | 470.3 ± 2.6 * |
| HbA1c (%) | 4.2 ± 0.3 | 8.1 ± 1.1 * | 4.3 ± 0.9 | 8.3 ± 1.3 * |
| Kidney weight/body weight (mg/g) | 9 ± 4 | 13 ± 2.1 * | 8.7 ± 2.3 | 9.3 ± 3.4 # |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tung, C.-W.; Ho, C.; Hsu, Y.-C.; Huang, S.-C.; Shih, Y.-H.; Lin, C.-L. MicroRNA-29a Attenuates Diabetic Glomerular Injury through Modulating Cannabinoid Receptor 1 Signaling. Molecules 2019, 24, 264. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24020264
Tung C-W, Ho C, Hsu Y-C, Huang S-C, Shih Y-H, Lin C-L. MicroRNA-29a Attenuates Diabetic Glomerular Injury through Modulating Cannabinoid Receptor 1 Signaling. Molecules. 2019; 24(2):264. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24020264
Chicago/Turabian StyleTung, Chun-Wu, Cheng Ho, Yung-Chien Hsu, Shun-Chen Huang, Ya-Hsueh Shih, and Chun-Liang Lin. 2019. "MicroRNA-29a Attenuates Diabetic Glomerular Injury through Modulating Cannabinoid Receptor 1 Signaling" Molecules 24, no. 2: 264. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24020264