Sequential Annulations to Interesting Novel Pyrrolo[3,2-c]carbazoles
Abstract
:1. Introduction
2. Results
2.1. Search for the Best Experimental Conditions
2.2. Verifying the Scope
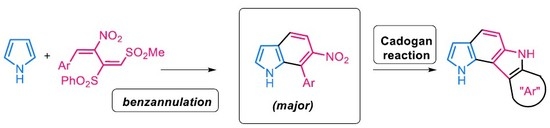
2.3. A Possible Elaboration of 7-Aryl-6-nitroindole 6 to Pyrrolo[3,2-c]carbazoles
2.4. Reduction of the Nitro- to Amino Group in Compounds 6: A First Step to Further Elaborations
3. Mechanistic Aspect Discussion
4. Materials and Methods
4.1. Substrates
4.2. General Procedure for the Synthesis of 7-arylsubstituted indoles (6–8 a–h)
4.3. General Procedure for the Cadogan Reaction: Synthesis of Pyrrolo[3,2-c]carbazoles 10
4.4. Procedure for the Reduction of the Nitro- to Amino- Group in Compound 6a
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gribble, G.W. Indole Ring Synthesis: From Natural Products to Drug Discovery; Wiley: Chichester, UK, 2016. [Google Scholar]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical importance of indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef] [PubMed]
- Heterocyclic scaffolds II: Reactions and applications of indoles. In Topics in Heterocyclic Chemistry; Gribble, G.W. (Ed.) Springer: Berlin/Heidelberg, Germany, 2010; Volume 26. [Google Scholar]
- Taber, D.F.; Tirunahari, P.K. Indole synthesis: A review and proposed classification. Tetrahedron 2011, 67, 7195–7210. [Google Scholar] [CrossRef] [PubMed]
- Lvov, A.G.; Kavun, A.M.; Kachala, V.V.; Lyssenko, K.A.; Shirinian, V.Z. Photorearrangement of dihetarylethenes as a tool for the benzannulation of heterocycles. Org. Biomol. Chem. 2019, 17, 4990–5000, and refs cited therein. [Google Scholar] [CrossRef]
- Barluenga, J.; Vazquez-Villa, H.; Ballesteros, A.; Gonzalez, J.M. Synthesis of Indoles upon Sequential Reaction of 3-Alkynylpyrrole-2-carboxaldehydes with iodonium ions and alkenes. Preparation of related benzofuran and benzothiophene derivatives. Adv. Synth. Catal. 2005, 347, 526–530. [Google Scholar] [CrossRef]
- Della Rosa, C.; Kneeteman, M.; Mancini, P. Comparison of the reactivity between 2-and 3-nitropyrroles in cycloaddition reactions. A simple indole synthesis. Tetrahedron Lett. 2007, 48, 1435–1438. [Google Scholar] [CrossRef]
- Giomi, D.; Cecchi, M. Study on direct benzoannelations of pyrrole and indole systems by domino reactions with 4,5-dicyanopyridazine. Tetrahedron 2002, 58, 8067–8071. [Google Scholar] [CrossRef]
- Ishibashi, H.; Tabata, T.; Hanaoka, K.; Iriyama, H.; Akamatsu, S.; Ikeda, M. A new, general entry to 4-substituted indoles. Synthesis of (S)-(−)-pindolol and (±)-chuangxinmycin. Tetrahedron Lett. 1993, 34, 489–492. [Google Scholar] [CrossRef]
- Liu, C.; Huang, W.; Wang, M.; Pan, B.; Gu, Y. expedient synthesis of substituted benzoheterocycles using 2-butoxy-2,3-dihydrofurans as [4 + 2] benzannulation reagents. Adv. Synth. Catal. 2016, 358, 2260–2266. [Google Scholar] [CrossRef]
- Lu, S.; Xu, R.; Li, Z. Benzannulation of pyrroles to 4,5-disubstituted indoles through brønsted-acid-promoted rearrangement of tert-butyl peroxides. Asian, J. Org. Chem. 2017, 6, 1604–1611. [Google Scholar] [CrossRef]
- Matsuda, Y.; Naoe, S.; Oishi, S.; Fujii, N.; Ohno, H. Formal [4 + 2] reaction between 1, 3-diynes and pyrroles: Gold (I)-catalyzed indole synthesis by double hydroarylation. Chem. Eur. J. 2015, 21, 1463–1467. [Google Scholar] [CrossRef]
- Dawande, S.G.; Kanchupalli, V.; Kalepu, J.; Chennamsetti, H.; Lad, B.S.; Katukojvala, S. Rhodium enalcarbenoids: Direct synthesis of indoles by rhodium (II)-catalyzed [4 + 2] benzannulation of pyrroles. Angew. Chem. Int. Ed. 2014, 53, 4076–4080. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.R.; Dilipkumar, U.; Reddy, M.D. Novel [4 + 2]-benzannulation to access substituted benzenes and polycyclic aromatic and benzene-fused heteroaromatic compounds. Org. Lett. 2014, 16, 3792–3795. [Google Scholar] [CrossRef] [PubMed]
- Thies, N.; Hrib, C.G.; Haak, E. Ruthenium-catalyzed functionalization of pyrroles and indoles with propargyl alcohols. Chem. Eur. J. 2012, 18, 6302–6308. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Maccagno, M.; Pani, M.; Petrillo, G.; Scapolla, C.; Tavani, C. A straight access to functionalized carbazoles by tandem reaction between indole and nitrobutadienes. Tetrahedron 2015, 71, 7421–7435. [Google Scholar] [CrossRef]
- Bianchi, L.; Giorgi, G.; Maccagno, M.; Petrillo, G.; Scapolla, C.; Tavani, C. An original route to newly-functionalized indoles and carbazoles starting from the ring-opening of nitrothiophenes. Tetrahedron Lett. 2012, 53, 752–757. [Google Scholar] [CrossRef]
- Bianchi, L.; Maccagno, M.; Petrillo, G.; Rizzato, E.; Sancassan, F.; Severi, E.; Spinelli, D.; Tavani, C.; Viale, M. Versatile nitrobutadienic building-blocks from the ring-opening of 2- and 3-nitrothiophenes. In Targets in Heterocyclic Systems: Chemistry and Properties; Attanasi, O.A., Spinelli, D., Eds.; Società Chimica Italiana: Rome, Italy, 2007; Volume 11, pp. 1–20. [Google Scholar]
- Bianchi, L.; Maccagno, M.; Petrillo, G.; Sancassan, F.; Spinelli, D.; Tavani, C. 2,3-Dinitro-1,3-butadienes: versatile building-blocks from the ring opening of 3,4-dinitrothiophene. In Targets in Heterocyclic Systems: Chemistry and Properties; Attanasi, O.A., Spinelli, D., Eds.; Società Chimica Italiana: Rome, Italy, 2006; Volume 10, pp. 1–23. [Google Scholar]
- Bianchi, L.; Dell’Erba, C.; Maccagno, M.; Morganti, S.; Petrillo, G.; Rizzato, E.; Sancassan, F.; Severi, E.; Spinelli, D.; Tavani, C. Nitrobutadienes from β-nitrothiophenes: Valuable building-blocks in the overall ring-opening/ring-closure protocol to homo- or hetero-cycles. Arkivoc 2006, 7, 169–185. [Google Scholar] [CrossRef]
- Pagano, A.; Mancinelli, M.; Bianchi, L.; Giorgi, G.; Maccagno, M.; Petrillo, G.; Tavani, C. Nitrobutadienes as powerful benzannulating agents: An unprecedented easy access to rare nitroindoles. Tetrahedron 2019, 75, 4506–4515. [Google Scholar] [CrossRef]
- Bouchard, J.; Wakim, S.; Leclerc, M. Synthesis of diindolocarbazoles by cadogan reaction: Route to ladder oligo(p-aniline)s. J. Org. Chem. 2004, 69, 5705–5711. [Google Scholar] [CrossRef]
- Giraud, F.; Pereira, E.; Anizon, F.; Moreau, P. Synthesis and applications of dihydropyrrolocarbazoles. Eur. J. Org. Chem. 2019, 5025–5042. [Google Scholar] [CrossRef]
- Akué-Gédu, R.; Nauton, L.; Théry, V.; Bain, J.; Cohen, P.; Anizon, F.; Moreau, P. Synthesis, Pim kinase inhibitory potencies and in vitro antiproliferative activities of diversely substituted pyrrolo[2,3-a] carbazoles. Bioorg. Med. Chem. 2010, 18, 6865–6873. [Google Scholar] [CrossRef]
- Zhang, W.; Ready, J.M. Total synthesis of the dictyodendrins as an arena to highlight emerging synthetic technologies. Nat. Prod. Rep. 2017, 34, 1010–1034. [Google Scholar] [CrossRef] [PubMed]
- Gamble, A.B.; Garner, J.; Gordon, C.P.; O’Conner, S.M.J.; Keller, P.A. Aryl nitro reduction with iron powder or stannous chloride under ultrasonic irradiation. Synthetic Communications 2007, 37, 2777–2786. [Google Scholar] [CrossRef]
- Bianchi, L.; Giorgi, G.; Maccagno, M.; Petrillo, G.; Scapolla, C.; Tavani, C. On the behavior of bis(sulfonyl)nitrobutadienes towards primary amines: A convenient access to 1-alkyl-2-aryl-4-(phenylsulfonyl)pyrroles. Tetrahedron 2016, 72, 7050–7058. [Google Scholar] [CrossRef]
- Benzi, A.; Bianchi, L.; Giorgi, G.; Maccagno, M.; Petrillo, G.; Tavani, C. 2-Aryl-3-Vinyl substituted Imidazo[1,2-a]Pyridines and fluorescent polyheterocycles therefrom. Manuscript in preparation.
- Clive, D.L.J.; Li, Z.; Yu, M. Intramolecular conjugate displacement: A general route to hexahydroquinolizines, hexahydroindolizines, and related [m,n,0]-bicyclic structures with nitrogen at a bridgehead. J. Org. Chem. 2007, 72, 5608–5617. [Google Scholar] [CrossRef]
- Wang, L.; Prabhudas, B.; Clive, D.L.J.J. Formation of carbocycles by intramolecular conjugate displacement: Scope and mechanistic insights. J. Am. Chem. Soc. 2009, 131, 6003–6012. [Google Scholar] [CrossRef]
- Baldwin, J.E. Rules for ring closure. J. Chem. Soc. Chem. Commun. 1976, 734–736. [Google Scholar] [CrossRef]
- Cox, E.D.; Cook, J.M. The Pictet-Spengler condensation: A new direction for an old reaction. Chem. Rev. 1995, 95, 1797–1842. [Google Scholar] [CrossRef]
- Stöckigt, J.; Antonchick, A.P.; Wu, F.; Waldmann, H. The Pictet-Spengler reaction in nature and in organic chemistry. Angew. Chem. Int. Ed. 2011, 50, 8538–8564. [Google Scholar] [CrossRef]
- Bianchi, L.; Dell’Erba, C.; Maccagno, M.; Mugnoli, A.; Novi, M.; Petrillo, G.; Sancassan, F.; Tavani, C. Access to ring-fused homo- and heteroaromatic derivatives via an initial ring-opening of 3-nitro-4-(phenylsulfonyl)thiophene. J. Org. Chem. 2003, 68, 5254–5260. [Google Scholar] [CrossRef]
- Appukkuttan, P.; Van der Eycken, E.; Dehaen, W. Microwave-enhanced Cadogan cyclization: An easy access to the 2-substituted carbazoles and other fused heterocyclic systems. Synlett 2005, 127–133. [Google Scholar] [CrossRef]
Sample Availability: Samples of all compounds are available from the authors. |










| Entry | T (°C) | Mol Eq. of Pyrrole | time (h) |  6a (Yield %) |  7a (Yield %) |  8a (Yield %) | Overall Balance |
|---|---|---|---|---|---|---|---|
| 1 | 50 | 2 | 24 | 52 | 16 | 14 | 82% |
| 2 | 50 | 4 | 2.5 | 53 | 21 | 17 | 91% |
| 3 | 40 | 4 | 5 | 57 | 17 | 12 | 86% |
| 4 | 30 | 4 | 24 | 57 | 16 | 19 | 92% |
| 5 | 0 | 4 | 48 | - | - | - | 100% of 4a |
| Entry | Ar in 4 |  6 (Yields %) |  7 (Yields %) |  8 (Yields %) |  9 (Yields %) | Overall Balance |
|---|---|---|---|---|---|---|
| 1 | p-Tol: 4a | 6a: 57 | 7a: 17 | 8a: 12 | 9a: nq2 | 86% |
| 2 | Ph: 4b | 6b: 60 | 7b: 10 | 8b: 10 | 9b: -3 | 80% |
| 3 | p-MeOC6H4: 4c | 6c: 44 | 7c: 17 | 8c: 13 | 9c: -3 | 74% |
| 4 | m-ClC6H4: 4d | 6d: 53 | 7d: 11 | 8d: 18 | 9d: -3 | 81% |
| 5 | p-ClC6H4: 4e | 6e: 42 | 7e: 8 | 8e: 15 | 9e: 18 | 83% |
| 6 | p-MeSO2C6H4: 4f | 6f: 19 | 7f: nq2 | 8f: nq2 | 9f: nq2 | nq |
| 7 | 3,5-(CF3)2C6H3: 4g | 6g: 22 | 7g: 10 | 8g: 11 | 9g: 25 | 68% |
| 8 | 1-Naphthyl: 4h | 6h: 40 | 7h: 14 | 8h: 13 | 9h: 13 | 80% |
| 9 | 2-Thienyl:4 4i | 6i: 33 | 7i: 7 | 8i: 11 | 9i: 14 | 65% |
| Entry | Substrate | Product | Yield | |
|---|---|---|---|---|
| 1 |  | 6a |  | 10a: 75% |
| 2 |  | 6b |  | 10b: 50% |
| 3 |  | 6c |  | 10c: 53% |
| 4 |  | 6d |  | 10d and 10d’: 75% ca. 1:1 |
| 5 |  | 6e |  | 10e: 51% |
| 6 |  | 6h |  | 10h: 65% |
| 7 |  | 6i |  | 10i: 38% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benzi, A.; Bianchi, L.; Maccagno, M.; Pagano, A.; Petrillo, G.; Tavani, C. Sequential Annulations to Interesting Novel Pyrrolo[3,2-c]carbazoles. Molecules 2019, 24, 3802. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24203802
Benzi A, Bianchi L, Maccagno M, Pagano A, Petrillo G, Tavani C. Sequential Annulations to Interesting Novel Pyrrolo[3,2-c]carbazoles. Molecules. 2019; 24(20):3802. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24203802
Chicago/Turabian StyleBenzi, Alice, Lara Bianchi, Massimo Maccagno, Angela Pagano, Giovanni Petrillo, and Cinzia Tavani. 2019. "Sequential Annulations to Interesting Novel Pyrrolo[3,2-c]carbazoles" Molecules 24, no. 20: 3802. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24203802