Nanostructured Nickel Aluminate as a Key Intermediate for the Production of Highly Dispersed and Stable Nickel Nanoparticles Supported within Mesoporous Alumina for Dry Reforming of Methane
Abstract
:1. Introduction
2. Results and Discussion
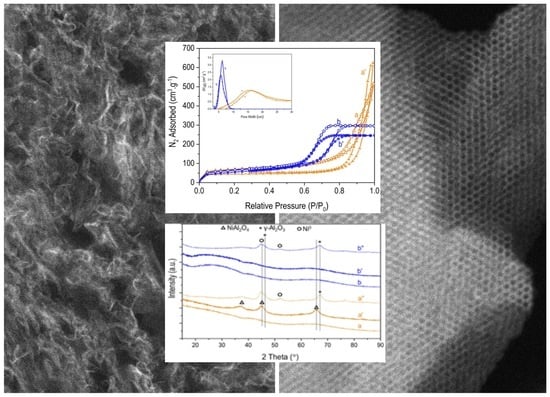
2.1. Mesoporous Organizations and Morphologies
2.2. Evidence of a Nickel Aluminate Spinel Phase in the Calcined Materials
2.3. Resulting Ni0 Dispersion and Catalytic Performance of the Reduced Catalysts
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El Hassan, N.; Kaydouh, M.N.; Geagea, H.; El Zein, H.; Jabbour, K.; Casale, S.; El Zakhem, H.; Massiani, P. Low temperature dry reforming of methane on rhodium and cobalt-based catalysts: Active phase stabilization by confinement in mesoporous SBA-15. Appl. Catal. A Gen. 2016, 520, 114–121. [Google Scholar] [CrossRef]
- Talyan, V.; Dahiya, R.P.; Anand, S.; Sreekrishnan, T.R. Quantification of methane emission from municipal solid waste disposal in Delhi. Resour. Conserv. Recycl. 2006, 50, 240–259. [Google Scholar] [CrossRef]
- Lavoie, J.; Labiano, F.G. Review of dry reforming of methane, a potentially more environmentally friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, R.; Hansen, J.B. CO2-reforming of methane over transition metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Verykios, X.E. Carbon dioxide reforming of methane to synthesis gas over supported Ni catalysts. Catal. Today 1994, 21, 589–595. [Google Scholar] [CrossRef]
- Karam, L.; El Hassan, N. Advantages of mesoporous silica-based catalysts in methane reforming by CO2 from kinetic perspective. J. Environ. Chem. Eng. 2018, 6, 4289–4297. [Google Scholar] [CrossRef]
- Mahoney, E.G.; Pusel, J.M.; Stagg-Williams, S.M.; Faraji, S. The effects of Pt addition to supported Ni catalysts on dry (CO2) reforming of methane to syngas. J. CO2 Util. 2014, 6, 40–44. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q.M.; Millar, G.J. Carbon Dioxide Reforming of Methane to Produce Synthesis Gas over Metal-Supported Catalysts: State of the Art. Energy Fuels 1996, 896–904. [Google Scholar] [CrossRef]
- Karam, L.; Casale, S.; El Zakhem, H.; El Hassan, N. Tuning the properties of nickel nanoparticles inside SBA-15 mesopores for enhanced stability in methane reforming. J. CO2 Util. 2017, 17, 119–124. [Google Scholar] [CrossRef]
- Karam, L.; Reboul, J.; Casale, S.; El Hassan, N.; Massiani, P. Porous nickel-alumina derived from metal-organic framework (MIL-53): A new approach to achieve active and stable catalysts in methane dry reforming. ChemCatChem 2019. [Google Scholar] [CrossRef]
- Jabbour, K.; Massiani, P.; Davidson, A.; Casale, S.; El Hassan, N. Ordered mesoporous “one-pot” synthesized Ni-Mg (Ca)-Al2O3 as effective and remarkably stable catalysts for combined steam and dry reforming of methane (CSDRM). Appl. Catal. B Environ. 2017, 201, 527–542. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Liu, Y.; Zhang, Y. Dry reforming of methane over Ni/MgO-Al2O3 catalysts prepared by two-step hydrothermal method. Appl. Surf. Sci. 2016, 389, 25–33. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Férey, G. A Rationale for the Large Breathing of the Porous Aluminum Terephthalate (MIL-53) Upon Hydration. Chem. A Eur. J. 2004, 10, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, M.K.; Sheibani, S.; Rashchi, F.; Gonzalez-Delacruz, V.M.; Caballero, A. Preparation of nanostructured nickel aluminate spinel powder from spent NiO/Al2O3 catalyst by mechano-chemical synthesis. Adv. Powder Technol. 2012, 23, 833–838. [Google Scholar] [CrossRef]
- Zhan, Y.; Han, J.; Bao, Z.; Cao, B.; Li, Y.; Street, J.; Yu, F. Biogas reforming of carbon dioxide to syngas production over Ni-Mg-Al catalysts. Mol. Catal. 2017, 436, 248–258. [Google Scholar] [CrossRef]
- Margossian, T.; Larmier, K.; Kim, S.M.; Krumeich, F.; Fedorov, A.; Chen, P.; Müller, C.R.; Copéret, C. Molecularly Tailored Nickel Precursor and Support Yield a Stable Methane Dry Reforming Catalyst with Superior Metal Use. J. Am. Chem. Soc. 2017, 139, 6919–6927. [Google Scholar] [CrossRef]
- Gonçalves, A.A.S.; Costa, M.J.F.; Zhang, L.; Ciesielczyk, F.; Jaroniec, M. One-Pot Synthesis of MeAl2O4 (Me = Ni, Co, or Cu) Supported on γ- Al2O3 with Ultralarge Mesopores: Enhancing Interfacial Defects in γ-Al2O3 to Facilitate the Formation of Spinel Structures at Lower Temperatures. Chem. Mater. 2018, 30, 436–446. [Google Scholar] [CrossRef]
- Fang, X.; Peng, C.; Peng, H.; Liu, W.; Xu, X.; Wang, X.; Li, C.; Zhou, W. Methane Dry Reforming over Coke-Resistant Mesoporous Ni-Al2O3 Catalysts Prepared by Evaporation-Induced Self-Assembly Method. ChemCatChem 2015, 7, 3753–3762. [Google Scholar] [CrossRef]
- Wang, N.; Shen, K.; Huang, L.; Yu, X.; Qian, W.; Chu, W. Facile route for synthesizing ordered mesoporous Ni-Ce-Al oxide materials and their catalytic performance for methane dry reforming to hydrogen and syngas. ACS Catal. 2013, 3, 1638–1651. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, H.; Song, H.; Chou, L. Ordered mesoporous alumina supported nickel-based catalysts for carbon dioxide reforming of methane. Int. J. Hydrogen Energy 2012, 37, 7497–7511. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Baklavaridis, A.; Papadakis, V.G.; Goula, M.A. Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al2O3 and Ni/CaO-Al2O3 catalysts. Waste Biomass Valorization 2016, 7, 725–736. [Google Scholar] [CrossRef]
- Hou, Z.; Yashima, T. Meso-porous Ni/Mg/Al catalysts for methane reforming with CO2. Appl. Catal. A Gen. 2004, 261, 205–209. [Google Scholar] [CrossRef]
- Le Saché, E.; Pastor-Pérez, L.; Watson, D.; Sepúlveda-Escribano, A.; Reina, T.R. Ni stabilised on inorganic complex structures: superior catalysts for chemical CO2 recycling via dry reforming of methane. Appl. Catal. B Environ. 2018, 236, 458–465. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Tarasov, A.L.; Chernyshev, V.V.; Kustov, L.M. Control of morphology and size of microporous framework MIL-53(Al) crystals by synthesis procedure. Mendeleev Commun. 2015, 25, 466–467. [Google Scholar] [CrossRef]
- Morris, S.M.; Fulvio, P.F.; Jaroniec, M. Ordered mesoporous alumina-supported metal oxides. J. Am. Chem. Soc. 2008, 130, 15210–15216. [Google Scholar] [CrossRef]
- Yuan, Q.; Yin, A.-X.; Luo, C.; Sun, L.-D.; Zhang, Y.-W.; Duan, W.-T.; Liu, H.-C.; Yan, C.-H. Facile Synthesis for Ordered Mesoporous γ-Aluminas with High Thermal Stability. J. Am. Chem. Soc. 2008, 130, 3465–3472. [Google Scholar] [CrossRef]







| Samples | Textural Properties of Calcined and Reduced (in Italic) Materials a | Temperature of Reduction b (°C) | Ni° Nanoparticle c Size (nm) | Catalytic Results d | |||||
|---|---|---|---|---|---|---|---|---|---|
| S.A. (m2·g−1) | Vtot (cm3·g−1) | Ø (nm) | Reduced | Spent | Conversions (%) | H2/CO Molar Ratio | |||
| XCH4 | XCO2 | ||||||||
| Ni5wt%-Al2O3-MOF | 240 (200) | 0.82 (1.02) | 10 (16) | 828 | 6.8 | 7.5 | 76 | 79 | 1.03 |
| Ni5wt%-Al2O3-EISA | 229 (196) | 0.49 (0.40) | 6.2 (5.7) | 708 | 7 | - | 71 | 75 | 1.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karam, L.; Reboul, J.; El Hassan, N.; Nelayah, J.; Massiani, P. Nanostructured Nickel Aluminate as a Key Intermediate for the Production of Highly Dispersed and Stable Nickel Nanoparticles Supported within Mesoporous Alumina for Dry Reforming of Methane. Molecules 2019, 24, 4107. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24224107
Karam L, Reboul J, El Hassan N, Nelayah J, Massiani P. Nanostructured Nickel Aluminate as a Key Intermediate for the Production of Highly Dispersed and Stable Nickel Nanoparticles Supported within Mesoporous Alumina for Dry Reforming of Methane. Molecules. 2019; 24(22):4107. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24224107
Chicago/Turabian StyleKaram, Leila, Julien Reboul, Nissrine El Hassan, Jaysen Nelayah, and Pascale Massiani. 2019. "Nanostructured Nickel Aluminate as a Key Intermediate for the Production of Highly Dispersed and Stable Nickel Nanoparticles Supported within Mesoporous Alumina for Dry Reforming of Methane" Molecules 24, no. 22: 4107. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24224107