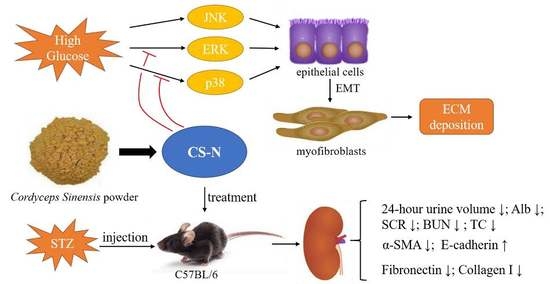
A Nucleoside/Nucleobase-Rich Extract from Cordyceps Sinensis Inhibits the Epithelial–Mesenchymal Transition and Protects against Renal Fibrosis in Diabetic Nephropathy
Abstract
:1. Introduction
2. Results
2.1. Analysis of Nucleosides and Nucleobases in CS-N
2.2. Effects of CS-N on Body Weights and Renal Function Parameters
2.3. The Effects of CS-N on Histopathological Changes in Renal Tissue
2.4. The Effects of CS-N on EMT and ECM Accumulation
2.5. CS-N Inhibited EMT and ECM Accumulation through p38 and ERK MAPK Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Isolation and Purification of CS-N
4.3. Analysis of Nucleosides and Nucleobases in CS-N
4.4. Animal Studies.
4.5. Cell Culture and Treatment
4.6. Renal Function Assessment
4.7. Histological Examination of the Kidneys
4.8. Immunohistochemical Analysis
4.9. Morphological Observations of HK-2 Cells
4.10. RT-PCR Analysis
4.11. Western Blot Analysis
4.12. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, S.S.; Kim, J.H.; Kim, I.J. Current Challenges in Diabetic Nephropathy: Early Diagnosis and Ways to Improve Outcomes. Endocrinol. Metab. 2016, 31, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, G.; Cooper, M.E. Diabetic nephropathy: Important pathophysiologic mechanisms. Diabetes Res. Clin. Pract. 2008, 82 (Suppl. 1), S75–S79. [Google Scholar] [CrossRef] [PubMed]
- Brenneman, J.; Hill, J.; Pullen, S. Emerging therapeutics for the treatment of diabetic nephropathy. Bioorgan. Med. Chem. Lett. 2016, 26, 4394–4402. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, I.; Wolf, G. Epithelial-to-Mesenchymal Transition in Diabetic Nephropathy: Fact or Fiction? Cells 2015, 4, 631–652. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chi, L.; Zhao, J.; Wang, X.; Chen, Z.; Meng, L.; Liu, G.; Guan, G.; Wang, F. Serum response factor provokes epithelial-mesenchymal transition in renal tubular epithelial cells of diabetic nephropathy. Physiol. Genom. 2016, 48, 580–588. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Zhang, Q.; Zhao, T. Low molecular weight fucoidan and its fractions inhibit renal epithelial mesenchymal transition induced by TGF-β1 or FGF-2. Int. J. Biol. Macromol. 2017, 105, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Liu, H.; Zhang, D.; Liu, Y.; Wang, C.; Liu, F.; Chen, J. HMGB1 Enhances the AGE-Induced Expression of CTGF and TGF-beta via RAGE-Dependent Signaling in Renal Tubular Epithelial Cells. Am. J. Nephrol. 2015, 41, 257–266. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Q.; Lai, S.; Yang, L.; Shi, G.; Wang, Q.; Luo, Z.; Zhao, R.; Yu, Y. Attenuation of diabetic nephropathy by Sanziguben Granule inhibiting EMT through Nrf2-mediated anti-oxidative effects in streptozotocin (STZ)-induced diabetic rats. J. Ethnopharmacol. 2017, 205, 207–216. [Google Scholar] [CrossRef]
- Zhao, D.; Jia, J.; Shao, H. miR-30e targets GLIPR-2 to modulate diabetic nephropathy: In vitro and in vivo experiments. J. Mol. Endocrinol. 2017, 59, 181–190. [Google Scholar] [CrossRef]
- Chen, P.X.; Wang, S.; Nie, S.; Marcone, M. Properties of Cordyceps Sinensis: A review. J. Funct. Foods 2013, 5, 550–569. [Google Scholar] [CrossRef]
- Zhang, H.W.; Lin, Z.X.; Tung, Y.S.; Kwan, T.H.; Mok, C.K.; Leung, C.; Chan, L.S. Cordyceps sinensis (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane Database Syst. Rev. 2014, 18, CD008353. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Weinberg, J.M.; Kriz, W.; Bidani, A.K. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J. Am. Soc. Nephrol. 2015, 26, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Dubey, N.K.; Li, W.S.; Liu, M.C.; Chiang, H.S.; Leu, S.J.; Shieh, Y.H.; Tsai, F.C.; Deng, W.P. Cordyceps militaris Treatment Preserves Renal Function in Type 2 Diabetic Nephropathy Mice. PLoS ONE 2016, 11, e0166342. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Jing, T.; Meng, Q.; Liu, C.; Hu, S.; Ma, Y.; Liu, Y.; Lu, J.; Cheng, Y.; Wang, D.; et al. Studies on the antidiabetic activities of Cordyceps militaris extract in diet-streptozotocin-induced diabetic Sprague-Dawley rats. BioMed Res. Int. 2014, 2014, 160980. [Google Scholar] [CrossRef]
- Xiao, J.H.; Qi, Y.; Xiong, Q. Nucleosides, a valuable chemical marker for quality control in traditional Chinese medicine Cordyceps. Recent Pat. Biotechnol. 2013, 7, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Peng, L.; Chen, H.; Shen, Y.; Yu, K.; Cheng, S. Cordycepin prevented IL-beta-induced expression of inflammatory mediators in human osteoarthritis chondrocytes. Int. Orthop. 2014, 38, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, J.; Zhang, Q.; Chen, P.; Song, J.; Yu, S.; Liu, H.; Liu, F.; Song, C.; Yang, D.; et al. Adenosine induces apoptosis in human liver cancer cells through ROS production and mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 2014, 448, 8–14. [Google Scholar] [CrossRef]
- Gu, L.; Johno, H.; Nakajima, S.; Kato, H.; Takahashi, S.; Katoh, R.; Kitamura, M. Blockade of Smad signaling by 3’-deoxyadenosine: A mechanism for its anti-fibrotic potential. Lab. Investig. J. Tech. Methods Pathol. 2013, 93, 450–461. [Google Scholar] [CrossRef]
- Asakura, M.; Asanuma, H.; Kim, J.; Liao, Y.; Nakamaru, K.; Fujita, M.; Komamura, K.; Isomura, T.; Furukawa, H.; Tomoike, H.; et al. Impact of adenosine receptor signaling and metabolism on pathophysiology in patients with chronic heart failure. Hypertens. Res. 2007, 30, 781–787. [Google Scholar] [CrossRef]
- Antonioli, L.; Blandizzi, C.; Csoka, B.; Pacher, P.; Hasko, G. Adenosine signalling in diabetes mellitus—Pathophysiology and therapeutic considerations. Nat. Rev. Endocrinol. 2015, 11, 228–241. [Google Scholar] [CrossRef]
- Peleli, M.; Carlstrom, M. Adenosine signaling in diabetes mellitus and associated cardiovascular and renal complications. Mol. Asp. Med. 2017, 55, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Merighi, S.; Borea, P.A.; Gessi, S. Adenosine receptors and diabetes: Focus on the A(2B) adenosine receptor subtype. Pharmacol. Res. 2015, 99, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.K.; Cheng, D.; Jackson, T.C.; Verrier, J.D.; Gillespie, D.G. Extracellular guanosine regulates extracellular adenosine levels. Am. J. Physiol. Cell Physiol. 2013, 304, C406–C421. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.K.; Cheng, D.; Mi, Z.; Gillespie, D.G. Guanosine regulates adenosine levels in the kidney. Physiol. Rep. 2014, 2, e12028. [Google Scholar] [CrossRef]
- Li, S.P.; Li, P.; Lai, C.M.; Gong, Y.X.; Kan, K.K.W.; Dong, T.T.X.; Tsim, K.W.K.; Wang, Y.T. Simultaneous determination of ergosterol, nucleosides and their bases from natural and cultured Cordyceps by pressurised liquid extraction and high-performance liquid chromatography. J. Chromatogr. A 2004, 1036, 239–243. [Google Scholar] [CrossRef]
- Yang, F.Q.; Guan, J.; Li, S.P. Fast simultaneous determination of 14 nucleosides and nucleobases in cultured Cordyceps using ultra-performance liquid chromatography. Talanta 2007, 73, 269–273. [Google Scholar] [CrossRef]
- Gong, Y.X.; Li, S.P.; Li, P.; Liu, J.J.; Wang, Y.T. Simultaneous determination of six main nucleosides and bases in natural and cultured Cordyceps by capillary electrophoresis. J. Chromatogr. A 2004, 1055, 215–221. [Google Scholar] [CrossRef]
- Gao, J.L.; Leung, K.S.; Wang, Y.T.; Lai, C.M.; Li, S.P.; Hu, L.F.; Lu, G.H.; Jiang, Z.H.; Yu, Z.L. Qualitative and quantitative analyses of nucleosides and nucleobases in Ganoderma spp. by HPLC-DAD-MS. J. Pharm. Biomed. Anal. 2007, 44, 807–811. [Google Scholar] [CrossRef]
- Tao, W.W.; Duan, J.A.; Yang, N.Y.; Guo, S.; Zhu, Z.H.; Tang, Y.P.; Qian, D.W. Determination of nucleosides and nucleobases in the pollen of Typha angustifolia by UPLC-PDA-MS. Phytochem. Anal. 2012, 23, 373–378. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.-Y.; Li, H.-M.; Wan, D.-J.; Tang, Y.-J. Determination of the nucleosides and nucleobases in Tuber samples by dispersive solid-phase extraction combined with liquid chromatography–mass spectrometry. Anal. Chim. Acta 2011, 687, 159–167. [Google Scholar] [CrossRef]
- Lenoir, O.; Jasiek, M.; Henique, C.; Guyonnet, L.; Hartleben, B.; Bork, T.; Chipont, A.; Flosseau, K.; Bensaada, I.; Schmitt, A.; et al. Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy 2015, 11, 1130–1145. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Kang, H.; Oh, Y.S.; Jun, H.S. Psoralea corylifolia L. Seed Extract Attenuates Diabetic Nephropathy by Inhibiting Renal Fibrosis and Apoptosis in Streptozotocin-Induced Diabetic Mice. Nutrients 2017, 9, 828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez Gutierrez, R.M.; Garcia Campoy, A.H.; Paredes Carrera, S.P.; Muniz Ramirez, A.; Mota Flores, J.M.; Flores Valle, S.O. 3’-O-beta-d-glucopyranosyl-alpha,4,2’,4’,6’-pentahydroxy-dihydrochalcone, from Bark of Eysenhardtia polystachya Prevents Diabetic Nephropathy via Inhibiting Protein Glycation in STZ-Nicotinamide Induced Diabetic Mice. Molecules 2019, 24, 1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Zhang, X.; Zhang, C.; Shen, Q.; Sun, G.; Sun, X. Notoginsenoside R1 Protects db/db Mice against Diabetic Nephropathy via Upregulation of Nrf2-Mediated HO-1 Expression. Molecules 2019, 24, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Z.M.; Wang, Q.; Wan, Q.; Lin, J.G.; Hu, M.S.; Liu, Y.X.; Wang, R. The role of the p38 MAPK signaling pathway in high glucose-induced epithelial-mesenchymal transition of cultured human renal tubular epithelial cells. PLoS ONE 2011, 6, e22806. [Google Scholar] [CrossRef]
- Kanasaki, K.; Taduri, G.; Koya, D. Diabetic nephropathy: The role of inflammation in fibroblast activation and kidney fibrosis. Front. Endocrinol. 2013, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jafari, M.; Dadras, F.; Ghadimipour, H.R.; Seif Rabiei, M.A.; Khoshjou, F. Tempol effect on epithelial-mesenchymal transition induced by hyperglycemia. J. Nephropathol. 2017, 6, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yin, Z.; Li, H.; Fan, J.; Yang, S.; Chen, C.; Wang, D.W. MiR-30c protects diabetic nephropathy by suppressing epithelial-to-mesenchymal transition in db/db mice. Aging Cell 2017, 16, 387–400. [Google Scholar] [CrossRef]
- Fragiadaki, M.; Mason, R.M. Epithelial-mesenchymal transition in renal fibrosis—evidence for and against. Int. J. Exp. Pathol. 2011, 92, 143–150. [Google Scholar] [CrossRef]
- Ravindran, S.; Kuruvilla, V.; Wilbur, K.; Munusamy, S. Nephroprotective Effects of Metformin in Diabetic Nephropathy. J. Cell. Physiol. 2017, 232, 731–742. [Google Scholar] [CrossRef]
- Kang, M.K.; Park, S.H.; Choi, Y.J.; Shin, D.; Kang, Y.H. Chrysin inhibits diabetic renal tubulointerstitial fibrosis through blocking epithelial to mesenchymal transition. J. Mol. Med. 2015, 93, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.J. Diabetic nephropathy: HIF activation in prevention of diabetic nephropathy. Nat. Rev. Nephrol. 2014, 10, 612. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, F.; Yang, S.; Chen, B.; Shi, J. Protocatechuic acid ameliorates high glucose-induced extracellular matrix accumulation in diabetic nephropathy. Biomed. Pharmacother. 2018, 98, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, X.; Tian, F.; Li, J.; Wang, Q.; Gu, C. MKP2 inhibits TGF-beta1-induced epithelial-to-mesenchymal transition in renal tubular epithelial cells through a JNK-dependent pathway. Clin. Sci. 2018, 132, 2339–2355. [Google Scholar] [CrossRef]
- Chuang, S.T.; Kuo, Y.H.; Su, M.J. Antifibrotic effects of KS370G, a caffeamide derivative, in renal ischemia-reperfusion injured mice and renal tubular epithelial cells. Sci. Rep. 2014, 4, 5814. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Shi, Y.; Hou, Y.; Ren, Y.; Du, C.; Zhang, L.; Li, Y.; Duan, H. Knockdown of thioredoxin-interacting protein ameliorates high glucose-induced epithelial to mesenchymal transition in renal tubular epithelial cells. Cell. Signal. 2013, 25, 2788–2796. [Google Scholar] [CrossRef]
- Hung, T.W.; Tsai, J.P.; Lin, S.H.; Lee, C.H.; Hsieh, Y.H.; Chang, H.R. Pentraxin 3 Activates JNK Signaling and Regulates the Epithelial-To-Mesenchymal Transition in Renal Fibrosis. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2016, 40, 1029–1038. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Xue, H.; Wang, Z.; Ni, J.; Yao, T.; Huang, Y.; Yu, C.; Lu, L. Angiotensin-(1-7) attenuates high glucose-induced proximal tubular epithelial-to-mesenchymal transition via inhibiting ERK1/2 and p38 phosphorylation. Life Sci. 2012, 90, 454–462. [Google Scholar] [CrossRef]
- Vaneckova, I.; Kujal, P.; Huskova, Z.; Vanourkova, Z.; Vernerova, Z.; Certikova Chabova, V.; Skaroupkova, P.; Kramer, H.J.; Tesar, V.; Cervenka, L. Effects of combined endothelin A receptor and renin-angiotensin system blockade on the course of end-organ damage in 5/6 nephrectomized Ren-2 hypertensive rats. Kidney Blood Press. Res. 2012, 35, 382–392. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






| Compounds | tR (min) | [M + H]+ (m/z) | MS2 (m/z) | DP (V) | CE (eV) |
|---|---|---|---|---|---|
| Cytidine | 3.69 | 244.2 | 111.7 | 50 | 15 |
| Adenine | 4.44 | 136.4 | 118.6 | 50 | 33 |
| Guanine | 5.09 | 152.2 | 134.7 | 100 | 32 |
| Uracil | 6.98 | 114.0 | 69.3 | 120 | 27 |
| Hypoxanthine | 9.57 | 137.2 | 109.3 | 150 | 39 |
| Uridine | 11.12 | 245.2 | 112.7 | 60 | 14 |
| 2’-Deoxyadenosine | 11.53 | 252.3 | 135.6 | 60 | 21 |
| Guanosine | 11.53 | 284.2 | 151.8 | 60 | 12 |
| Adenosine | 11.55 | 268.2 | 135.7 | 60 | 22 |
| Thymidine | 11.62 | 243.2 | 126.5 | 30 | 13 |
| Compounds | Content (mg/g) | Compounds | Content (mg/g) |
|---|---|---|---|
| Cytidine | 2.81 ± 0.21 | Uridine | 150.70 ± 2.65 |
| Adenine | 6.91 ± 0.32 | Adenosine | 41.64 ± 1.16 |
| Guanine | 95.84 ± 0.58 | 2’-Deoxyadenosine | 13.02 ± 0.78 |
| Uracil | 74.12 ± 1.07 | Guanosine | 194.91 ± 2.39 |
| Hypoxanthine | 37.82 ± 1.42 | Thymidine | 11.89 ± 0.44 |
| Week | NC | NC + CS-N | DN | DN + ENA | DN + CS-N40 | DN + CS-N80 |
|---|---|---|---|---|---|---|
| 0 | 22.02 ± 1.14 | 21.42 ± 0.85 | 21.62 ± 0.94 | 21.6 ± 1.56 | 21.28 ± 0.76 | 21.63 ± 0.84 |
| 1 | 23.54 ± 1.14 | 22.65 ± 0.81 | 18.84 ± 1.23 * | 18.91 ± 1.64 | 18.40 ± 1.39 | 19.17 ± 2.02 |
| 2 | 24.24 ± 1.36 | 23.67 ± 0.93 | 18.55 ± 1.89 * | 19.84 ± 1.55 | 19.21 ± 1.42 | 19.97 ± 2.09 |
| 3 | 25.64 ± 1.70 | 24.37 ± 1.34 | 18.33 ± 2.12 * | 20.63 ± 1.48 | 19.96 ± 1.31 | 20.40 ± 2.10 |
| 4 | 26.22 ± 1.53 | 25.07 ± 1.39 | 18.41 ± 2.48 * | 20.38 ± 1.51 | 19.92 ± 1.52 | 21.07 ± 1.47 # |
| 5 | 26.69 ± 1.55 | 25.67 ± 1.58 | 18.73 ± 2.25 * | 20.72 ± 1.39 | 20.72 ± 1.54 | 21.28 ± 1.65 # |
| 6 | 27.09 ± 2.23 | 26.13 ± 1.45 | 17.39 ± 2.44 * | 20.28 ± 2.12 | 20.43 ± 1.36 # | 21.17 ± 2.54 # |
| 7 | 27.88 ± 2.22 | 26.57 ± 1.40 | 17.13 ± 2.05 * | 20.61 ± 2.08 | 20.42 ± 1.82 # | 20.73 ± 2.50 # |
| 8 | 28.38 ± 2.27 | 26.97 ± 1.78 | 17.27 ± 1.68 * | 20.33 ± 2.17 # | 20.59 ± 1.83 # | 20.83 ± 2.53 # |
| Week | NC | NC + CS-N | DN | DN + ENA | DN + CS-N40 | DN + CS-N80 |
|---|---|---|---|---|---|---|
| 1 | 6.15 ± 0.38 | 5.80 ± 0.75 | 26.03 ± 2.37 * | 26.77 ± 1.90 | 27.20 ± 2.47 | 25.80 ± 3.06 |
| 3 | 6.20 ± 0.24 | 6.05 ± 0.55 | 27.82 ± 1.52 * | 27.27 ± 1.65 | 27.17 ± 2.29 | 27.78 ± 1.87 |
| 5 | 5.83 ± 0.36 | 6.07 ± 0.58 | 27.62 ± 1.67 * | 26.97 ± 1.87 | 26.05 ± 2.67 | 27.00 ± 1.06 |
| 7 | 6.03 ± 0.52 | 6.13 ± 0.45 | 27.50 ± 0.95 * | 26.30 ± 0.92 | 26.80 ± 1.34 | 26.73 ± 1.06 |
| Name | Accession Number | Primer |
|---|---|---|
| Fibronectin | NM_212482.3 | Forward: 5′-TGGAGGAAGCCGAGGTTT-3′ Reverse: 5′-CAGCGGTTTGCGATGGTA-3′ |
| Collagen I | NM_000088.3 | Forward: 5′-GTGCGATGACGTGATCTGT-3′ Reverse: 5′-TTGGTCGGTGGGTGACTCT-3′ |
| β-actin | NM_001101.5 | Forward: 5′-ACTCTTCCAGCCTTCCTTCC-3′ Reverse: 5′-GAGGAGCAATGATCTTGATCTTC-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Sun, Y.; Wei, G.; Li, S.; Zhao, Z. A Nucleoside/Nucleobase-Rich Extract from Cordyceps Sinensis Inhibits the Epithelial–Mesenchymal Transition and Protects against Renal Fibrosis in Diabetic Nephropathy. Molecules 2019, 24, 4119. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24224119
Dong Z, Sun Y, Wei G, Li S, Zhao Z. A Nucleoside/Nucleobase-Rich Extract from Cordyceps Sinensis Inhibits the Epithelial–Mesenchymal Transition and Protects against Renal Fibrosis in Diabetic Nephropathy. Molecules. 2019; 24(22):4119. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24224119
Chicago/Turabian StyleDong, Zhonghua, Yueyue Sun, Guangwei Wei, Siying Li, and Zhongxi Zhao. 2019. "A Nucleoside/Nucleobase-Rich Extract from Cordyceps Sinensis Inhibits the Epithelial–Mesenchymal Transition and Protects against Renal Fibrosis in Diabetic Nephropathy" Molecules 24, no. 22: 4119. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24224119