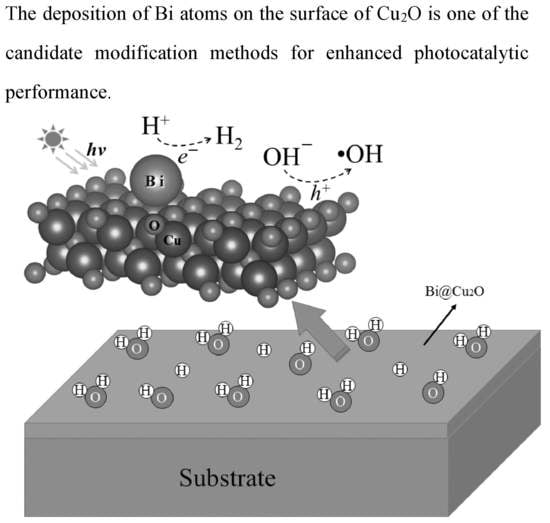
Passivating Surface States on Water Splitting Cuprous Oxide Photocatalyst with Bismuth Decoration
Abstract
:1. Introduction
2. Computational Detail
3. Results and Discussion
3.1. Surface Property
3.2. Adsorption Energy
3.3. Electronic Structure
3.4. Band Edge
3.5. Optical Property
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, H.G.; Zhu, Q.H.; Zhang, Y.; Wang, Y.; Zhao, L.; Yu, B. One-pot synthesis and hierarchical assembly of hollow Cu2O microspheres with nanocrystals-composed porous multishell and their gas-sensing properties. Adv. Funct. Mater. 2007, 17, 2766–2771. [Google Scholar] [CrossRef]
- Wu, L.; Wu, Y.L.; Jin, S.J.; Zhang, L.; Xun, Z.P. Gas sensitivity and photocatalytic performance of cuprous oxide with novel morphologies. Chem. Phys. Lett. 2016, 662, 47–51. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Tian, F.H.; Chen, S.G.; Ma, Z.Q.; Zhao, L.H.; Jia, X.F. Density functional theory study on the mechanism of CO sensing on Cu2O(111) surface: Influence of the pre-adsorbed oxygen atom. Appl. Surf. Sci. 2014, 288, 452–457. [Google Scholar] [CrossRef]
- Wang, Y.C.; Qin, C.; Lou, Z.R.; Lu, Y.F.; Zhu, L.P. Cu2O photocathodes for unassisted solar water-splitting devices enabled by noble-metal cocatalysts simultaneously as hydrogen evolution catalysts and protection layers. Nanotechnology 2019, 30, 495407. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.F.; Kim, J.H.; Mayer, M.T.; Son, M.K.; Ummadisingu, A.; Lee, J.S.; Hagfeldt, A.; Luo, J.S.; Gratzel, M. Boosting the performance of Cu2O photocathodes for unassisted solar water splitting devices. Nat. Catal. 2018, 1, 412–420. [Google Scholar] [CrossRef]
- Niu, W.Z.; Cai, T.M.W.; Joliat, R.W.; Zhu, L.P.; Tilley, S.D. Extended Light Harvesting with Dual Cu2O-Based Photocathodes for High Efficiency Water Splitting. Adv. Energy Mater. 2017, 8, 1702323. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef]
- Yu, X.J.; Zhang, J.; Niu, J.F.; Zhao, J.; Wei, Y.C.; Yao, B.H. Photocatalytic degradation of ciprofloxacin using Zn-doped Cu2O particles: Analysis of degradation pathways and intermediates. Chem. Eng. J. 2019, 374, 316–327. [Google Scholar] [CrossRef]
- Zhan, B.; Liu, Y.; Li, S.Y.; Kaya, C.; Stegmaier, T.; Aliabadi, M.; Han, Z.W.; Ren, L.Q. Fabrication of superwetting Cu@Cu2O cubic film for oil/water emulsion separation and photocatalytic degradation. Appl. Surf. Sci. 2019, 496, 143580. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, H.K.; Chen, H. Enhanced visible light photocatalytic activity of Cu2O via cationic-anionic passivated codoping. Phys. Chem. Chem. Phys. 2015, 17, 630–637. [Google Scholar] [CrossRef]
- Tang, L.L.; Du, Y.H.; Kong, C.C.; Sun, S.D.; Yang, Z.M. One-pot synthesis of etched Cu2O cubes with exposed 110 facets with enhanced visible-light-driven photocatalytic activity. Phys. Chem. Chem. Phys. 2015, 17, 29479–29482. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.D. Recent advances in hybrid Cu2O-based heterogeneous nanostructures. Nanoscale 2015, 7, 10850–10882. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, J.Y.; Wang, R.M.; Wang, M. Highly efficient hydrogen production and formaldehyde degradation by Cu2O microcrystals. Appl. Catal. B Environ. 2015, 238, 37–38. [Google Scholar] [CrossRef]
- Hara, M.; Kondo, T.; Komoda, M.; Ikeda, S.; Shinohara, K.; Tanaka, A.; Kondo, J.N.; Domen, K. Cu2O as a photocatalyst for overall water splitting under visible light irradiation. Chem. Commun. 1998, 4, 357–358. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Sun, L.L.; Wang, G.H.; Hao, R.R.; Han, D.Y.; Cao, S. Solvothermal fabrication and enhanced visible light photocatalyticactivity of Cu2O-reduced graphene oxide composite microspheres for photodegradation of Rhodamine B. Appl. Surf. Sci. 2015, 358, 91–99. [Google Scholar] [CrossRef]
- Cai, J.Y.; Liu, W.J.; Li, Z.H. One-pot self-assembly of Cu2O/RGO composite aerogel for aqueousphotocatalysis. Appl. Surf. Sci. 2015, 358, 146–151. [Google Scholar] [CrossRef]
- Liu, Z.F.; Yan, L. High-efficiency p-n junction oxide photoelectrodes for photoelectrochemical water splitting. Phys. Chem. Chem. Phys. 2016, 18, 31230–31237. [Google Scholar] [CrossRef]
- Xu, L.; Xu, H.Y.; Wu, S.B.; Zhang, X.Y. Synergy effect over electrodeposited submicron Cu2O films in photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2012, 258, 4934–4938. [Google Scholar] [CrossRef]
- Bendavid, L.I.; Carter, E.A. First-Principles Predictions of the structure, stability, and photocatalytic potential of Cu2O surfaces. J. Phys. Chem. B 2013, 117, 15750–15760. [Google Scholar] [CrossRef]
- Wang, G.Z.; Chen, H.; Li, Y.; Kuang, A.L.; Yuan, H.K.; Wu, G. A hybrid density functional study on the visible light photocatalytic activity of (Mo,Cr)-N codoped KNbO3. Phys. Chem. Chem. Phys. 2015, 17, 28743–28753. [Google Scholar] [CrossRef]
- Li, Y.F.; Yin, W.J.; Deng, R.; Chen, R.; Chen, J.; Yan, Q.Y.; Yao, B.; Sun, H.D.; Wei, S.; Wu, T. Realizing a SnO2-based ultraviolet light-emitting diode via breaking the dipole-forbidden rule. NPG Asia Mater. 2012, 4, e30. [Google Scholar] [CrossRef]
- Meyer, B.K.; Polity, A.; Reppin, D.; Becker, M.; Hering, P.; Klar, P.J.; Sander, T.; Reindl, C.; Benz, J.; Eickhoff, M.; et al. Binary copper oxide semiconductors: From materials towards devices. Phys. Status Solidi B 2012, 249, 1487–1509. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Chanda, K.; Chu, Y.T.; Chiu, C.Y.; Huang, M.H. Direct formation of small Cu2O nanocubes, octahedra, and octapods for efficient synthesis of triazoles. Nanoscale 2014, 6, 8704–8709. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Rej, S.; Hsu, S.C. Facet-dependent properties of polyhedral nanocrystals. Chem. Commun. 2014, 50, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.A.; Bedford, N.M.; Ren, Y.; Zahran, E.M.; Goodin, R.C.; Chagani, F.F.; Bachas, L.G.; Knecht, M.R. Direct Synthetic Control over the Size, Composition, and Photocatalytic Activity of Octahedral Copper Oxide Materials: Correlation Between Surface Structure and Catalytic Functionality. ACS Appl. Mater. Interfaces 2015, 7, 13238–13250. [Google Scholar] [CrossRef]
- Li, S.M.; Ge, X.; Jiang, S.N.; Peng, X.N.; Zhang, Z.; Li, W.X.; Yu, S.S. Synthesis of octahedral and cubic Cu2O microcrystals in sub- and super-critical methanol and their photocatalytic performance. J. Mater. Sci. 2015, 50, 4115–4121. [Google Scholar] [CrossRef]
- Liang, Y.H.; Shang, L.; Bian, T.; Zhou, C.; Zhang, D.H.; Yu, H.J.; Xu, H.T.; Shi, Z.; Zhang, T.R.; Wu, L.Z.; et al. Shape-controlled synthesis of polyhedral 50-facet Cu2O microcrystals with high-index facets. CrystEngComm 2012, 14, 4431–4436. [Google Scholar] [CrossRef]
- Sun, S.D.; Yang, Z.M. Recent advances in tuning crystal facets of polyhedral cuprous oxide architectures. RSC Adv. 2014, 4, 3804–3822. [Google Scholar] [CrossRef]
- Kuo, C.H.; Huang, M.H. Morphologically controlled synthesis of Cu2O nanocrystals and their properties. Nano Today 2010, 5, 106–116. [Google Scholar] [CrossRef]
- Hua, Q.; Shang, D.L.; Zhang, W.H.; Chen, K.; Chang, S.J.; Ma, Y.S.; Jiang, Z.Q.; Yang, J.L.; Huang, W.X. Morphological Evolution of Cu2O Nanocrystals in an Acid Solution: Stability of Different Crystal Planes. Langmuir 2011, 27, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Ulman, K.; Nguyen, M.T.; Seriani, N.; Gebauer, R. Passivation of surface states of α-Fe2O3(0001) surface by deposition of Ga2O3 overlayers: A density functional theory study. J. Chem. Phys. 2016, 144, 094701. [Google Scholar] [CrossRef] [PubMed]
- Le Fomal, F.; Tetreault, N.; Cornuz, M.; Moehl, T.; Grätzel, M.; Sivula, K. Passivating surface states on water splitting hematite photoanodes with alumina overlayers. Chem. Sci. 2011, 2, 737–743. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, Z.; Spurgeon, J.; Yang, X.G. Enhanced photoelectrochemical water-splitting performance of semiconductors by surface passivation layers. Energy Environ. Sci. 2014, 7, 2504–2517. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew-Burke-Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef] [Green Version]
- White, J.A.; Bird, D.M. Implementation of gradient-corrected exchange-correlation potentials in Car-Parrinello total-energy calculations. Phys. Rev. B 1994, 50, 4954–4957. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillonin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef] [Green Version]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Erratum: Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2006, 118, 8207–8215, Correction in 2006, 124, 219906. [Google Scholar] [CrossRef] [Green Version]
- Soon, A.; Todorova, M.; Delley, B.; Stampfl, C. Thermodynamic stability and structure of copper oxide surfaces: A first-principles investigation. Phys. Rev. B 2007, 75, 125420. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.K.; Chen, H.; Kuang, A.L.; Miao, Y.; Xiong, Z.H. Density-functional study of small neutral and cationic bismuth clusters and (n = 2–24). J. Chem. Phys. 2008, 128, 094305. [Google Scholar] [CrossRef] [PubMed]
- Artrith, N.; Sailuam, W.; Limpijumnong, S.; Kolpak, A.M. Reduced overpotentials for electrocatalytic water splitting over Fe- and Ni-modified BaTiO3. Phys. Chem. Chem. Phys. 2016, 18, 29561–29570. [Google Scholar] [CrossRef]
- Chakrapani, V.; Angus, J.C.; Anderson, A.B.; Wolter, S.D.; Stoner, B.R.; Sumanasekera, G.U. Charge Transfer Equilibria Between Diamond and an Aqueous Oxygen Electrochemical Redox Couple. Science 2007, 318, 1424–1430. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Sinha, T.P. Electronic structure, chemical bonding, and optical properties of paraelectric BaTiO3. Phys. Rev. B 2000, 62, 8828–8834. [Google Scholar] [CrossRef]
- Tian, F.H.; Liu, C.B. DFT Description on Electronic Structure and Optical Absorption Properties of Anionic S-Doped Anatase TiO2. J. Chem. Phys. B 2006, 110, 17866–17871. [Google Scholar] [CrossRef]
- Fu, Q.; He, T.; Li, J.L.; Yang, G.W. Band-engineered SrTiO3 nanowires for visible light photocatalysis. J. Appl. Phys. 2012, 112, 104322. [Google Scholar] [CrossRef]








| Systems | Adsorption Site | |||
|---|---|---|---|---|
| (ML) | (eV/Å2) | (ev/Å2) | ||
| Cu2O(100) | 0 | - | - | −2.01 |
| p (2 × 2) | 0.25 | O-top | −0.04 | −2.05 |
| p (2 × 2) | 0.25 | O-Bridge | −0.07 | −2.08 |
| p (2 × 2) | 0.25 | Cu1-top | −0.05 | −2.06 |
| p (2 × 2) | 0.25 | Cu2-top | −0.05 | −2.06 |
| p (2 × 2) | 0.25 | Hollow | −0.08 | −2.09 |
| p (3 × 3) | 0.11 | Hollow | −0.04 | −2.05 |
| p (3 × 3) | 0.22 | Hollow | −0.08 | −2.09 |
| p (3 × 3) | 0.33 | Hollow | −0.09 | −2.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Yuan, H.; Chen, H. Passivating Surface States on Water Splitting Cuprous Oxide Photocatalyst with Bismuth Decoration. Molecules 2019, 24, 4156. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24224156
Huang Y, Yuan H, Chen H. Passivating Surface States on Water Splitting Cuprous Oxide Photocatalyst with Bismuth Decoration. Molecules. 2019; 24(22):4156. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24224156
Chicago/Turabian StyleHuang, Yuhong, Hongkuan Yuan, and Hong Chen. 2019. "Passivating Surface States on Water Splitting Cuprous Oxide Photocatalyst with Bismuth Decoration" Molecules 24, no. 22: 4156. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24224156