Analysis of the Heterogeneous Distribution of Amiloride and Propranolol in Dried Blood Spot by UHPLC-FLD and MALDI-IMS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Model Compounds
2.2. Sample Treatment and Recovery Calculation
- Whole blood spots were extracted using 200 µL of the extractant (the same volume used for punched samples), analyzed, and compared to a standard solution of 0.125 mg/L, which would be the expected concentration if the recovery was 100%.
- Whole blood spots were extracted using 500 µL of the extractant in order to keep the same sample area/extractant volume ratio used for punched samples, as the area of the whole blood is 2.5 times the area of the punch. These samples were analyzed and compared to a standard solution of 0.05 mg/L.
- Punched blood spots taken from the center of the drop were extracted using 200 µL of the extractant, analyzed, and compared to a standard solution of 0.05 mg/L, which would be the concentration in the extractant supposing a 100% recovery and a homogeneous distribution of the analyte and the blood in the DBS.
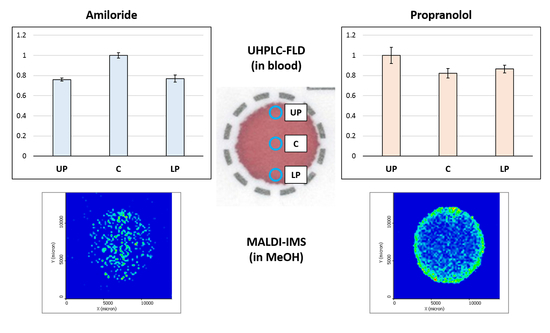
2.3. Study of the Distribution of the Analytes in the DBS
2.3.1. Impact of the Hematocrit and Study of the Distribution of the Analyte in DBS Samples by HPLC-FLD
2.3.2. Study of the Distribution of the Analytes in the DBS Support by MALDI-IMS
3. Materials and Methods
3.1. Reagents and Solutions
3.2. Instrumentation
3.3. Blood Samples
3.4. Chromatographic Conditions
3.5. MALDI-IMS Analysis Conditions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Odoardi, S.; Anzillotti, L.; Strano-Rossi, S. Simplifying sample pretreatment: Application of dried blood spot (DBS) method to blood samples, including postmortem, for UHPLC-MS/MS analysis of drugs of abuse. Forensic Sci. Int. 2014, 243, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Tretzel, L.; Thomas, A.; Geyer, H.; Gmeiner, G.; Forsdahl, G.; Pop, V.; Schanzer, W.; Thevis, M. Use of dried blood spots in doping control analysis of anabolic steroid esters. J. Pharm. Biomed. Anal. 2014, 96, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Tonoli, D.; Varesio, E.; Hopfgartner, G. The use of mass spectrometry to analyze dried blood spots. Mass Spectrom. Rev. 2016, 35, 361–438. [Google Scholar] [CrossRef] [PubMed]
- Demirev, P.A. Dried blood spots: Analysis and applications. Anal. Chem. 2013, 85, 779–789. [Google Scholar] [CrossRef]
- Guthrie, R.; Susi, A. A Simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 1963, 32, 338–343. [Google Scholar]
- Hawwa, A.F.; Albawab, A.; Rooney, M.; Wedderburn, L.R.; Beresford, M.W.; McElnay, J.C. A novel dried blood spot-LCMS method for the quantification of methotrexate polyglutamates as a potential marker for methotrexate use in children. PLoS ONE 2014, 9, e89908. [Google Scholar] [CrossRef]
- Deglon, J.; Thomas, A.; Mangin, P.; Staub, C. Direct analysis of dried blood spots coupled with mass spectrometry: Concepts and biomedical applications. Anal. Bioanal. Chem. 2012, 402, 2485–2498. [Google Scholar] [CrossRef]
- Enderle, Y.; Foerster, K.; Burhenne, J. Clinical feasibility of dried blood spots: Analytics, validation, and applications. J. Pharm. Biomed. Anal. 2016, 130, 231–243. [Google Scholar] [CrossRef]
- Na-Bangchang, K.; Guirou, E.A.; Cheomung, A.; Karbwang, J. Determination of Primaquine in Whole Blood and Finger-Pricked Capillary Blood Dried on Filter Paper Using HPLC and LCMS/MS. Chromatographia 2014, 77, 561–569. [Google Scholar] [CrossRef]
- Ter Heine, R.; Rosing, H.; van Gorp, E.C.; Mulder, J.W.; van der Steeg, W.A.; Beijnen, J.H.; Huitema, A.D. Quantification of protease inhibitors and non-nucleoside reverse transcriptase inhibitors in dried blood spots by liquid chromatography-triple quadrupole mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 867, 205–212. [Google Scholar] [CrossRef]
- Vu, D.H.; Koster, R.A.; Alffenaar, J.W.; Brouwers, J.R.; Uges, D.R. Determination of moxifloxacin in dried blood spots using LC-MS/MS and the impact of the hematocrit and blood volume. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Meesters, R.J.W. Biofluid collection in metabolomics by the application of the novel volumetric absorptive microsampling technology: A mini-review. Rev. Sep. Sci. 2019, 1, 34–46. [Google Scholar] [CrossRef]
- Velghe, S.; Delahaye, L.; Stove, C.P. Is the hematocrit still an issue in quantitative dried blood spot analysis? J. Pharm. Biomed. Anal. 2019, 163, 188–196. [Google Scholar] [CrossRef]
- Li, W.; Lee, M.S. Dried Blood Spots: Applications and Techniques; Wiley: Hoboken, NJ, USA, 2014; p. 376. [Google Scholar]
- Cobb, Z.; de Vries, R.; Spooner, N.; Williams, S.; Staelens, L.; Doig, M.; Broadhurst, R.; Barfield, M.; van de Merbel, N.; Schmid, B.; et al. In-depth study of homogeneity in DBS using two different techniques: Results from the EBF DBS-microsampling consortium. Bioanalysis 2013, 5, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- De Kesel, P.M.; Capiau, S.; Lambert, W.E.; Stove, C.P. Current strategies for coping with the hematocrit problem in dried blood spot analysis. Bioanalysis 2014, 6, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Capiau, S.; Stove, V.V.; Lambert, W.E.; Stove, C.P. Prediction of the hematocrit of dried blood spots via potassium measurement on a routine clinical chemistry analyzer. Anal. Chem. 2013, 85, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.D.; Sennbro, C.J.; Svanstrom, C.; Hansson, G.P. Applying dried blood spot sampling with LCMS quantification in the clinical development phase of tasquinimod. Bioanalysis 2015, 7, 179–191. [Google Scholar] [CrossRef]
- Wilhelm, A.J.; den Burger, J.C.; Vos, R.M.; Chahbouni, A.; Sinjewel, A. Analysis of cyclosporin A in dried blood spots using liquid chromatography tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 1595–1598. [Google Scholar] [CrossRef]
- Li, Y.; Henion, J.; Abbott, R.; Wang, P. The use of a membrane filtration device to form dried plasma spots for the quantitative determination of guanfacine in whole blood. Rapid Commun. Mass Spectrom. 2012, 26, 1208–1212. [Google Scholar] [CrossRef]
- Hall, E.M.; Flores, S.R.; De Jesús, V.R. Influence of Hematocrit and Total-Spot Volume on Performance Characteristics of Dried Blood Spots for Newborn Screening. Int. J. Neonatal Screen. 2015, 1, 69–78. [Google Scholar] [CrossRef]
- Li, W.; Tse, F.L. Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Biomed. Chromatogr. 2010, 24, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Berm, E.J.; Paardekooper, J.; Brummel-Mulder, E.; Hak, E.; Wilffert, B.; Maring, J.G. A simple dried blood spot method for therapeutic drug monitoring of the tricyclic antidepressants amitriptyline, nortriptyline, imipramine, clomipramine, and their active metabolites using LC-MS/MS. Talanta 2015, 134, 165–172. [Google Scholar] [CrossRef] [PubMed]
- De Vries, R.; Barfield, M.; van de Merbel, N.; Schmid, B.; Siethoff, C.; Ortiz, J.; Verheij, E.; van Baar, B.; Cobb, Z.; White, S.; et al. The effect of hematocrit on bioanalysis of DBS: Results from the EBF DBS-microsampling consortium. Bioanalysis 2013, 5, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Cox, H.D.; Rampton, J.; Eichner, D. Quantification of insulin-like growth factor-1 in dried blood spots for detection of growth hormone abuse in sport. Anal. Bioanal. Chem. 2013, 405, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.V.; Henion, J.; Wickremsinhe, E. Fully-Automated Approach for Online Dried Blood Spot Extraction and Bioanalysis by Two-Dimensional-Liquid Chromatography Coupled with High-Resolution Quadrupole Time-of-Flight Mass Spectrometry. Anal. Chem. 2014, 86, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rabie, P.; Denniff, P.; Spooner, N.; Chowdhry, B.Z.; Pullen, F.S. Investigation of different approaches to incorporating internal standard in DBS quantitative bioanalytical workflows and their effect on nullifying hematocrit-based assay bias. Anal. Chem. 2015, 87, 4996–5003. [Google Scholar] [CrossRef] [PubMed]
- La Marca, G.; Malvagia, S.; Materazzi, S.; Della Bona, M.L.; Boenzi, S.; Martinelli, D.; Dionisi-Vici, C. LC-MS/MS method for simultaneous determination on a dried blood spot of multiple analytes relevant for treatment monitoring in patients with tyrosinemia type I. Anal. Chem. 2012, 84, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Holub, M.; Tuschl, K.; Ratschmann, R.; Strnadova, K.A.; Muhl, A.; Heinze, G.; Sperl, W.; Bodamer, O.A. Influence of hematocrit and localisation of punch in dried blood spots on levels of amino acids and acylcarnitines measured by tandem mass spectrometry. Clin. Chim. Acta 2006, 373, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Paehler, T.; Zimmer, M.; Guo, Z.; Zane, P.; Emmons, G.T. Impact of various factors on radioactivity distribution in different DBS papers. Bioanalysis 2010, 2, 1469–1475. [Google Scholar] [CrossRef]
Sample Availability: Not available. |




| Amiloride (%) | Propranolol (%) | |
|---|---|---|
| (1) Whole spot +200 µL extractant | 60 ± 2 | 77 ± 3 |
| (2) Whole spot + 500 µL extractant | 70 ± 1 | 88 ± 2 |
| (3) Punch + 200 µL extractant | 95 ± 3 | 85 ± 7 |
| Analyte | Volume (µL) | p-Value (Before Area Correction) | p-Value (After Area Correction) |
|---|---|---|---|
| Amiloride | 15 | 0.00917 | 0.000186 |
| 25 | 0.33358 | 0.042743 | |
| 35 | 0.10356 | 0.000010 | |
| Propranolol | 15 | 0.001917 | 0.079917 |
| 25 | 0.000200 | 0.589000 | |
| 35 | 0.000009 | 0.546900 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uribe, B.; González, O.; Blanco, M.E.; Albóniga, O.E.; Alonso, M.L.; Alonso, R.M. Analysis of the Heterogeneous Distribution of Amiloride and Propranolol in Dried Blood Spot by UHPLC-FLD and MALDI-IMS. Molecules 2019, 24, 4320. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24234320
Uribe B, González O, Blanco ME, Albóniga OE, Alonso ML, Alonso RM. Analysis of the Heterogeneous Distribution of Amiloride and Propranolol in Dried Blood Spot by UHPLC-FLD and MALDI-IMS. Molecules. 2019; 24(23):4320. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24234320
Chicago/Turabian StyleUribe, Beatriz, Oskar González, María Encarnación Blanco, Oihane Elena Albóniga, María Luz Alonso, and Rosa María Alonso. 2019. "Analysis of the Heterogeneous Distribution of Amiloride and Propranolol in Dried Blood Spot by UHPLC-FLD and MALDI-IMS" Molecules 24, no. 23: 4320. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24234320