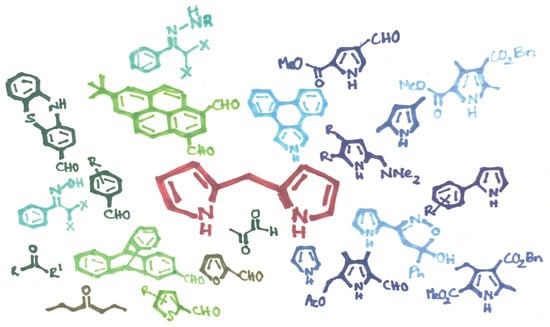
Current Advances in the Synthesis of Valuable Dipyrromethane Scaffolds: Classic and New Methods
Abstract
:1. Introduction
2. Classic Synthetic Strategies
2.1. Hydrochloric Acid-Catalyzed Dipyrromethane Synthesis
2.2. Acetic/propionic Acid-Catalyzed Dipyromethane Synthesis
2.3. p-Toluenesulphonic Acid-Catalyzed Dipyrromethane Synthesis
2.4. Trifluoroacetic Acid-Catalyzed Dipyrromethane Synthesis
2.5. Boron Trifluoride Diethyl Etherate-Catalyzed Dipyrromethane Synthesis
2.6. Indium(III) Chloride-Catalyzed Dipyrromethane Synthesis
2.7. Other Strategies
3. Novel Synthetic Strategies
3.1. Dipyrromethane Synthesis from Aldehydes and Pyrroles
3.2. Dipyrromethane Synthesis via Alternative Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lindsey, J.S. Synthetic routes to meso-patterned porphyrins. Acc. Chem. Res. 2010, 43, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Yedukondalu, M.; Ravikanth, M. Core-modified porphyrin based assemblies. Coord. Chem. Rev. 2011, 255, 547–573. [Google Scholar] [CrossRef]
- Gale, P.A.; Anzenbacher, P.; Sessler, J.S. Calixpyrroles II. Coord. Chem. Rev. 2001, 222, 57–102. [Google Scholar]
- Taniguchi, M.; Lindsey, J.S. Synthetic chlorins, possible surrogates for chlorophylls, prepared by derivatization of porphyrins. Chem. Rev. 2017, 117, 344–535. [Google Scholar] [CrossRef]
- Orłowski, R.; Gryko, D.; Gryko, D.T. Synthesis of corroles and their heteroanalogs. Chem. Rev. 2017, 117, 3102–3137. [Google Scholar] [CrossRef]
- Pareek, Y.; Ravikanth, M.; Chandrashekar, T.K. Smaragdyrins: Emeralds of expanded porphyrin family. Acc. Chem. Res. 2012, 45, 1801–1816. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.; Srinivasan, A.; Ravikanth, M.; Chandrashekar, T.K. Smaragdyrins and sapphyrins analogues. Chem. Rev. 2017, 117, 3329–3376. [Google Scholar] [CrossRef]
- Wood, T.E.; Thompson, A. Advances in the chemistry of dipyrrins and their complexes. Chem. Rev. 2007, 107, 1831–1861. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.C.; Hall, M.J. Recent developments in the synthesis of the BODIPY dyes. In Advances in Heterocyclic Chemistry; Eric, F.V., Scriven, C.A.R., Eds.; Elsevier: Amsterdam, the Netherlands, 2019; Volume 128, pp. 181–261. [Google Scholar]
- Loudet, A.; Burgess, K. BODIPY® dyes and their derivatives: Syntheses and spectroscopic properties. In Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific: Hackensack, NJ, USA, 2010; Volume 8, pp. 1–164. [Google Scholar]
- Gianapoulos, C.G.; Kirschbaum, K.; Mason, M.R. Mono- and bimetallic aluminum alkyl, aryl, and hydride complexes of a bulky dipyrromethene ligand. Organometallics 2014, 33, 4503–4511. [Google Scholar] [CrossRef]
- Gianapoulos, C.G.; Kumar, A.; Zhao, Y.; Jia, L.; Kirschbaum, K.; Mason, M.R. Aluminum alkoxide, amide and halide complexes supported by a bulky dipyrromethene ligand: Synthesis, characterization, and preliminary ε-caprolactone polymerization activity. Dalton Trans. 2016, 45, 13787–13797. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Halper, S.R. Dipyrromethene complexes of iron. Inorg. Chim. Acta 2002, 341, 12–16. [Google Scholar] [CrossRef]
- King, E.R.; Betley, T.A. C-H bond amination from a ferrous dipyrromethene complex. Inorg. Chem. 2009, 48, 2361–2363. [Google Scholar] [CrossRef] [PubMed]
- King, E.R.; Hennessy, E.T.; Betley, T.A. Catalytic C-H bond amination from high-spin iron imido complexes. J. Am. Chem. Soc. 2011, 133, 4917–4923. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Parkin, S.; Ozerov, O.V. Double C-H activation results in ruthenium complexes of a neutral PCP ligand with a central carbene moiety. Organometallics 2006, 25, 5345–5354. [Google Scholar] [CrossRef]
- Swavey, S.; DeBeer, M.; Li, K. Photoinduced interactions of supramolecular ruthenium(II) complexes with plasmid DNA: Synthesis and spectroscopic, electrochemical, and DNA photocleavage studies. Inorg. Chem. 2015, 54, 3139–3147. [Google Scholar] [CrossRef]
- Majumder, S.; Odom, A.L. Group-4 dipyrrolylmethane complexes in intramolecular olefin hydroamination. Organometallics 2008, 27, 1174–1177. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, A.K.; Pandey, R.; Pandey, D.S. Synthesis and characterization of complexes imparting N-pyridyl bonded meso-pyridyl substituted dipyrromethanes. J. Organometall. Chem. 2010, 695, 841–849. [Google Scholar] [CrossRef]
- Yin, Z.; Yan, Y.; Sun, S.; Wang, W. Syntheses, structures, and properties of Ni(II) complexes with 5,5’-bis(4-halogenphenyl)diazo-dipyrromethane. J. Coord. Chem. 2012, 65, 865–874. [Google Scholar] [CrossRef]
- King, E.R.; Betley, T.A. Unusual electronic structure of first row transition metal complexes featuring redox-active dipyrromethane ligands. J. Am. Chem. Soc 2009, 131, 14374–14380. [Google Scholar] [CrossRef]
- Reid, S.D.; Wilson, C.; Blake, A.J.; Love, J.B. Tautomerisation and hydrogen-bonding interactions in four-coordinate metal halide and azide complexes of N-donor-extended dipyrromethanes. Dalton Trans. 2010, 39, 418–425. [Google Scholar] [CrossRef]
- Tamaru, S.-I.; Yu, L.; Youngblood, W.J.; Muthukumaran, K.; Taniguchi, M.; Lindsey, J.S. A tin-complexation strategy for use with diverse acylation methods in the preparation of 1,9-diacyldipyrromethanes. J. Org. Chem. 2004, 69, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, J.A.; Fernández, I.; García-Álvarez, P.; Laglera-Gándara, C.J. A dipyrromethane-based diphosphane–germylene as precursor to tetrahedral copper(I) and T-shaped silver(I) and gold(I) PGeP pincer complexes. Dalton Trans. 2019, 48, 13273–13280. [Google Scholar] [CrossRef] [PubMed]
- Alešković, M.; Basarić, N.; Mlinarić-Majerski, K.; Molčanov, K.; Kojić-Prodić, B.; Kesharwani, M.K.; Ganguly, B. Anion recognition through hydrogen bonding by adamantane-dipyrromethane receptors. Tetrahedron 2010, 66, 1689–1698. [Google Scholar] [CrossRef]
- You, J.-M.; Jeong, H.; Seo, H.; Jeon, S. A new fluoride ion colorimetric sensor based on dipyrrolemethanes. Sens. Actuators B Chem. 2010, 146, 160–164. [Google Scholar] [CrossRef]
- Kataev, E.A.; Müller, C.; Kolesnikov, G.V.; Khrustalev, V.N. Guanidinium-based artificial receptors for binding orthophosphate in aqueous solution. Eur. J. Org. Chem. 2014, 2014, 2747–2753. [Google Scholar] [CrossRef]
- Muwal, P.K.; Nayal, A.; Jaiswal, M.K.; Pandey, P.S. A dipyrromethane based receptor as a dual colorimetric sensor for F− and Cu2+ ions. Tetrahedron Lett. 2018, 59, 29–32. [Google Scholar] [CrossRef]
- Susmel, S.; Comuzzi, C. Selectivity and efficiency of conductive molecularly imprinted polymer (c-MIP) based on 5-phenyl-dipyrromethane and 5-phenol-dipyrromethane for quorum sensing precursors detection. Chemosensors 2017, 5, 5. [Google Scholar] [CrossRef]
- Pereira, N.A.M.; Pinho e Melo, T.M.V.D. Recent developments in the synthesis of dipyrromethanes. A review. Org. Prep.Proc. Int. 2014, 46, 183–213. [Google Scholar] [CrossRef]
- Gryko, D.T.; Gryko, D.; Lee, C.-H. 5-Substituted dipyrranes: Synthesis and reactivity. Chem. Soc. Rev. 2012, 41, 3780–3789. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Nagarkatti, J.P.; Ashley, K.R. Synthesis of pyridyl meso substituted dipyrrylmethanes. Synthesis 1974, 186–187. [Google Scholar] [CrossRef]
- Baskın, D.; Çetinkaya, Y.; Balci, M. Synthesis of dipyrrolo-diazepine derivatives via intramolecular alkyne cyclization. Tetrahedron 2018, 74, 4062–4070. [Google Scholar] [CrossRef]
- Swavey, S.; Li, K. A dimetallic osmium(II) complex as a potential phototherapeutic agent: Binding and photocleavage studies with plasmid DNA. Eur. J. Inorg. Chem. 2015, 2015, 5551–5555. [Google Scholar] [CrossRef]
- Vicente, M.G.H.; Smith, K.M. Syntheses and functionalizations of porphyrin macrocycles. Curr. Org. Synth. 2014, 11, 3–28. [Google Scholar] [CrossRef] [Green Version]
- Neya, S.; Yoneda, T.; Hoshino, T.; Kawaguchi, A.T.; Suzuki, M. Synthesis of type III isomers of diacetyldeutero-, hemato-, and protoporphyrins with the use of Knorr’s pyrrole. Tetrahedron 2016, 72, 4022–4026. [Google Scholar] [CrossRef]
- Neya, S.; Yoneda, T.; Omori, H.; Hoshino, T.; Kawaguchi, A.T.; Suzuki, M. Synthesis of 1,4,5,8-tetraethyl-2,3,6,7-tetravinylporphyrin from a Knorr’s pyrrole analogue. Tetrahedron 2017, 73, 6780–6785. [Google Scholar] [CrossRef]
- Singh, R.N.; Rawat, P.; Kumar, A.; Kant, P.; Srivastava, A. Spectral analysis, chemical reactivity and first hyperpolarizability evaluation of a novel 1,9–bis(2–cyano–2–ethoxycarbonylvinyl)–5–(2–furyl)–dipyrromethane: Experimental and theoretical approaches. Spectrosc. Lett. 2015, 48, 235–250. [Google Scholar] [CrossRef]
- Rawat, P.; Singh, R.N.; Niranjan, P.; Ranjan, A.; Holguín, N.R.F. Evaluation of antituberculosis activity and DFT study on dipyrromethane-derived hydrazone derivatives. J. Mol. Struct. 2017, 1149, 539–548. [Google Scholar] [CrossRef]
- Mahanta, S.P.; Panda, P.K. 5,10-Diacylcalix[4]pyrroles: Synthesis and anion binding studies. Org. Biomol. Chem. 2014, 12, 278–285. [Google Scholar] [CrossRef]
- Sen, P.; Yildiz, S.Z.; Atalay, Y.; Dege, N.; Demirtas, G. The synthesis, characterization, crystal structure and theoretical calculations of a new meso-BODIPY substituted phthalonitrile. J. Lumin. 2014, 149, 297–305. [Google Scholar] [CrossRef]
- Sen, P.; Atmaca, G.Y.; Erdoğmuş, A.; Dege, N.; Genç, H.; Atalay, Y.; Yildiz, S.Z. The synthesis, characterization, crystal structure and photophysical properties of a new meso-BODIPY substituted phthalonitrile. J. Fluoresc. 2015, 25, 1225–1234. [Google Scholar] [CrossRef]
- Radzuan, N.H.M.; Malek, N.H.A.; Ngatiman, M.F.; Xin, T.K.; Bakar, M.B.; Hassan, N.I.; Bakar, M.A. Synthesis and X-ray single crystal study of 5-(4,4,5,5–tetramethyl–1,3,2–dioxoborolane)-10,20–diphenylporphyrin. Sains Malays. 2018, 47, 2083–2090. [Google Scholar] [CrossRef]
- Umasekhar, B.; Ganapathi, E.; Chatterjee, T.; Ravikanth, M. Synthesis, structure, and spectral, electrochemical and fluoride sensing properties of meso-pyrrolyl boron dipyrromethene. Dalton Trans. 2015, 44, 16516–16527. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Banasz, R.; Kubicki, M.; Patroniak, V. Dipyrromethane functionalized monomers as precursors of electrochromic polymers. Electrochim. Acta 2017, 258, 571–581. [Google Scholar] [CrossRef]
- Swamy, C.A.; Pakkirisamy, T. Effect of substituent position on optical properties of boron-dipyrromethane isomers. Inorg. Chim. Acta 2014, 411, 97–101. [Google Scholar]
- Golf, H.R.A.; Reissig, H.-U.; Wiehe, A. Synthesis of 5-substituted tetrapyrroles, metalloporphyrins, BODIPYs, and their dipyrrane precursors. J. Org. Chem. 2015, 80, 5133–5143. [Google Scholar] [CrossRef]
- Gutsche, C.S.; Hohlfeld, B.F.; Flanagan, K.J.; Senge, M.O.; Kulak, N.; Wiehe, A. Sequential nucleophilic substitution of the α-pyrrole and p-aryl positions of meso-pentafluorophenyl-substituted BODIPYs. Eur. J. Org. Chem. 2017, 2017, 3187–3196. [Google Scholar] [CrossRef]
- Hohlfeld, B.F.; Flanagan, K.J.; Kulak, N.; Senge, M.O.; Christmann, M.; Wiehe, A. Synthesis of porphyrinoids, BODIPYs, and (dipyrrinato)ruthenium(II) complexes from prefunctionalized dipyrromethanes. Eur. J. Org. Chem. 2019, 2019, 4020–4033. [Google Scholar] [CrossRef]
- Deliomeroglu, M.K.; Lynch, V.M.; Sessler, J.L. Conformationally switchable non-cyclic tetrapyrrole receptors: Synthesis of tetrakis(1H-pyrrole-2-carbaldehyde) derivatives and their anion binding properties. Chem. Commun. 2014, 50, 11863–11866. [Google Scholar] [CrossRef]
- Deliomeroglu, M.K.; Lynch, V.M.; Sessler, J.L. Non-cyclic formylated dipyrromethanes as phosphate anion receptors. Chem. Sci. 2016, 7, 3843–3850. [Google Scholar] [CrossRef] [Green Version]
- Pankhurst, J.R.; Cadenbach, T.; Betz, D.; Finn, C.; Love, J.B. Towards dipyrrins: Oxidation and metalation of acyclic and macrocyclic Schiff-base dipyrromethanes. Dalton Trans. 2015, 44, 2066–2070. [Google Scholar] [CrossRef] [Green Version]
- Temelli, B.; Kalkan, H. Unexpected formation of β, meso-directly linked diporphyrins under Adler–Longo reaction conditions. Synth. Commun. 2018, 48, 2112–2117. [Google Scholar] [CrossRef]
- Basumatary, B.; Reddy, R.V.R.; Bhandary, S.; Sanka, J. Gallium(III)corrole–BODIPY hybrid: Novel photophysical properties and first observation of b–f⋯f interactions. Dalton Trans. 2015, 44, 20817–20821. [Google Scholar] [CrossRef]
- He, R.B.; Yue, H.; Kong, J.H. Covalent porphyrin hybrids linked with dipyrrin, bidipyrrin or thiacorrole. Molecules 2017, 22, 1400. [Google Scholar]
- Lakshmi, V.; Lee, W.-Z.; Ravikanth, M. Synthesis, structure and spectral and electrochemical properties of 3-pyrrolyl BODIPY-metal dipyrrin complexes. Dalton Trans. 2014, 43, 16006–16014. [Google Scholar] [CrossRef]
- Sharma, R.; Gobeze, H.B.; D’Souza, F.; Ravikanth, M. Panchromatic light capture and efficient excitation transfer leading to near-ir emission of BODIPY oligomers. ChemPhysChem 2016, 17, 2516–2524. [Google Scholar] [CrossRef]
- Brem, B.; Gal, E.; Gaina, L.; Cristea, C.; Silaghi-Dumitrescu, L. Synthesis of novel (phenothiazinyl)dipyrrolylmethanes. Revue Roumaine De Chimie 2014, 59, 947–952. [Google Scholar]
- Brem, B.; Gal, E.; Gaina, L.; Lovasz, T.; Molnar, E.A.; Porumb, D.; Cristea, C. Novel 1,9-diacyl-5-(phenothiazinyl)dipyrromethane dialkyltin complexes. Studia Universitatis Babes-Bolyai Chemia 2016, 61, 73–80. [Google Scholar]
- Swamy, C.A.; Priyanka, R.N.; Thilagar, P. Triarylborane–dipyrromethane conjugates bearing dual receptor sites: The synthesis and evaluation of the anion binding site preference. Dalton Trans. 2014, 43, 4067–4075. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Jonaghani, M.A.; Amini, M.; Mortazavi-Manesh, A. Synthesis of dipyrromethanes in water and investigation of electronic and steric effects in efficiency of olefin epoxidation by sodium periodate catalyzed by manganese tetraaryl and trans disubstituted porphyrin complexes. J. Porphyr. Phthalocyan. 2019, 23, 671–678. [Google Scholar] [CrossRef]
- Sobral, A.l.J.F.N.; Rebanda, N.G.C.L.; da Silva, M.; Lampreia, S.H.; Ramos Silva, M.; Beja, A.M.; Paixão, J.A.; Rocha Gonsalves, A.M.d.A. One-step synthesis of dipyrromethanes in water. Tetrahedron Lett. 2003, 44, 3971–3973. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Sun, S.; He, Q.; Lynch, V.M.; Sessler, J.L. Pyrene-linked formylated bis(dipyrromethane): A fluorescent probe for dihydrogen phosphate. Org. Lett. 2018, 20, 5414–5417. [Google Scholar] [CrossRef]
- Kumar, S.; Gobeze, H.B.; Chatterjee, T.; D’Souza, F.; Ravikanth, M. Directly connected azaBODIPY–BODIPY dyad: Synthesis, crystal structure, and ground- and excited-state interactions. J. Phys. Chem. A 2015, 119, 8338–8348. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Chatterjee, T.; Ravikanth, M. β-meso covalently linked azaBODIPY-Pd(II) dipyrrin conjugate. ChemistrySelect 2016, 1, 94–100. [Google Scholar] [CrossRef]
- Koch, A.; Ravikanth, M. Monofunctionalized 1,3,5,7-tetraarylazaBODIPYs and their application in the synthesis of azaBODIPY based conjugates. J. Org. Chem. 2019, 84, 10775–10784. [Google Scholar] [CrossRef]
- Epelde-Elezcano, N.; Palao, E.; Manzano, H.; Prieto-CastaÇeda, A.; Agarrabeitia, A.R.; Tabero, A.; Villanueva, A.; Moya, S.; López-Arbeloa, I.; Martínez-Martínez, V.; et al. Rational design of advanced photosensitizers based on orthogonal BODIPY dimers to finely modulate singlet oxygen generation. Chem. Eur. J. 2017, 23, 4837–4848. [Google Scholar] [CrossRef]
- Esipova, T.V.; Vinogradov, S.A. Synthesis of phosphorescent asymmetrically π-extended porphyrins for two-photon applications. J. Org. Chem. 2014, 79, 8812–8825. [Google Scholar] [CrossRef] [Green Version]
- Sahin, T.; Vairaprakash, P.; Borbas, K.E.; Balasubramanian, T.; Lindsey, J.S. Hydrophilic bioconjugatable trans-AB-porphyrins and peptide conjugates. J. Porphyr. Phthalocyan. 2015, 19, 663–678. [Google Scholar] [CrossRef]
- Smoleń, S.; Walaszek, D.J.; Karczewski, M.; Martin, E.; Gryko, D. Towards no-free regulation of sGC: Design and synthesis of trans-AB-porphyrins. Isr. J. Chem. 2016, 56, 156–168. [Google Scholar] [CrossRef]
- Emandi, G.; Shaker, Y.M.; Flanagan, K.J.; O’Brien, J.M.; Senge, M.O. Merging triptycene, BODIPY and porphyrin chemistry: Synthesis and properties of mono- and trisubstituted triptycene dye arrays. Eur. J. Org. Chem. 2017, 2017, 6680–6692. [Google Scholar] [CrossRef]
- Petrushenko, K.B.; Petrushenko, I.K.; Petrova, O.V.; Sobenina, L.N.; Ushakov, I.A.; Trofimov, B.A. Environment-responsive 8-CF3-BODIPY dyes with aniline groups at the 3 position: Synthesis, optical properties and RI-CC2 calculations. Asian J. Org. Chem. 2017, 6, 852–861. [Google Scholar] [CrossRef]
- Tomilin, D.N.; Sobenina, L.N.; Petrushenko, K.B.; Ushakov, I.A.; Trofimov, B.A. Design of novel meso-CF3-BODIPY dyes with isoxazole substituents. Dyes Pigm. 2018, 152, 14–18. [Google Scholar] [CrossRef]
- Kashi, C.; Wu, C.-C.; Mai, C.-L.; Yeh, C.-Y.; Chang, C.K. Synthesis of octafluoroporphyrin. Angew. Chem. Int. Ed. 2016, 55, 5035–5039. [Google Scholar] [CrossRef]
- Clezy, P.S.; Smythe, G.A. The chemistry of pyrrolic compounds. VIII. Dipyrrylthiones. Aust. J. Chem. 1969, 22, 239–249. [Google Scholar] [CrossRef]
- Rangaraj, P.; Parshamoni, S.; Konar, S. MOF as a syringe pump for the controlled release of iodine catalyst in the synthesis of meso-thienyl dipyrromethanes. Chem. Commun. 2015, 51, 15526–15529. [Google Scholar] [CrossRef]
- Megarajan, S.; Ayaz Ahmed, K.B.; Rajmohan, R.; Vairaprakash, P.; Anbazhagan, V. An easily accessible and recyclable copper nanoparticle catalyst for the solvent-free synthesis of dipyrromethanes and aromatic amines. RSC Adv. 2016, 6, 103065–103071. [Google Scholar] [CrossRef]
- Rajmohan, R.; Ayaz Ahmed, K.B.; Sangeetha, S.; Anbazhagan, V.; Vairaprakash, P. C–H oxidation and chelation of a dipyrromethane mediated rapid colorimetric naked-eye Cu(II) chemosensor. Analyst 2017, 142, 3346–3351. [Google Scholar] [CrossRef]
- Rajaswathi, K.; Jayanthi, M.; Rajmohan, R.; Anbazhagan, V.; Vairaprakash, P. Simple admixture of 4-nitrobenzaldehyde and 2,4-dimethylpyrrole for efficient colorimetric sensing of copper(II) ions. Spectrochim. Acta A 2019, 212, 308–314. [Google Scholar] [CrossRef]
- Jaratjaroonphong, J.; Tuengpanya, S.; Saeeng, R.; Udompong, S.; Srisook, K. Green synthesis and anti-inflammatory studies of a series of 1,1-bis(heteroaryl)alkane derivatives. Eur. J. Med. Chem. 2014, 83, 561–568. [Google Scholar] [CrossRef]
- Senapak, W.; Saeeng, R.; Jaratjaroonphong, J.; Kasemsuk, T.; Sirion, U. Green synthesis of dipyrromethanes in aqueous media catalyzed by SO3H-functionalized ionic liquid. Org. Biomol. Chem. 2016, 14, 1302–1310. [Google Scholar] [CrossRef]
- Rawat, A.K. Highly efficient synthesis of aryldipyrromethanes in presence of bronsted acidic ionic liquids. Heterocycl. Lett. 2018, 8, 43–47. [Google Scholar]
- Chatterjee, R.; Mahato, S.; Santra, S.; Zyryanov, G.V.; Hajra, A.; Majee, A. Imidazolium zwitterionic molten salt: An efficient organocatalyst under neat conditions at room temperature for the synthesis of dipyrromethanes as well as bis(indolyl)methanes. ChemistrySelect 2018, 3, 5843–5847. [Google Scholar] [CrossRef]
- Singhal, A.; Singh, S.; Chauhan, S.M.S. Synthesis of dipyrromethanes in aqueous media using boric acid. Arkivoc 2016, vi, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Pereira, N.A.M.; Lopes, S.M.M.; Lemos, A.; Pinho e Melo, T.M.V.D. On-water synthesis of dipyrromethanes via bis-hetero-diels-alder reaction of azo- and nitrosoalkenes with pyrrole. Synlett 2014, 25, 423–427. [Google Scholar] [CrossRef]
- Lopes, S.M.M.; Cardoso, A.L.; Lemos, A.; Pinho e Melo, T.M.V.D. Recent advances in the chemistry of conjugated nitrosoalkenes and azoalkenes. Chem. Rev. 2018, 118, 11324–11352. [Google Scholar] [CrossRef]
- Lopes, S.M.M.; Lemos, A.; Pinho e Melo, T.M.V.D. Reactivity of dipyrromethanes towards azoalkenes: Synthesis of functionalized dipyrromethanes, calix[4]pyrroles, and bilanes. Eur. J. Org. Chem. 2014, 2014, 7039–7048. [Google Scholar] [CrossRef]
- Nunes, S.C.C.; Lopes, S.M.M.; Gomes, C.S.B.; Lemos, A.; Pais, A.; Pinho e Melo, T.M.V.D. Reactions of nitrosoalkenes with dipyrromethanes and pyrroles: Insight into the mechanistic pathway. J. Org. Chem. 2014, 79, 10456–10465. [Google Scholar] [CrossRef]
- Jorda, R.; Lopes, S.M.M.; Řezníčková, E.; Kryštof, V.; Pinho e Melo, T.M.V.D. Biological evaluation of dipyrromethanes in cancer cell lines: Antiproliferative and pro-apoptotic properties. ChemMedChem 2017, 12, 701–711. [Google Scholar] [CrossRef]
- del Río, M.; Lobo, F.; López, J.C.; Oliden, A.; Bañuelos, J.; López-Arbeloa, I.; Garcia-Moreno, I.; Gómez, A.M. One-pot synthesis of rotationally restricted, conjugatable, BODIPY derivatives from phthalides. J. Org. Chem. 2017, 82, 1240–1247. [Google Scholar] [CrossRef]
- Xiong, R.; Borbas, K.E. Mild microwave-assisted synthesis of dipyrromethanes and their analogues. Synlett 2015, 26, 484–488. [Google Scholar]
- Lyubimova, T.V.; Syrbu, S.A.; Semeikin, A.S. Synthesis of porphyrins from alpha-unsubstituted dipyrromethanes. Macroheterocycles 2016, 9, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Melanson, J.A.; Smithen, D.A.; Cameron, T.S.; Thompson, A. Microwave-assisted reduction of F-BODIPYs and dipyrrins to generate dipyrromethanes. Can. J. Chem. 2014, 92, 688–694. [Google Scholar] [CrossRef]
- Li, R.; Lammer, A.D.; Ferrence, G.M.; Lash, T.D. Synthesis, structural characterization, aromatic characteristics, and metalation of neo-confused porphyrins, a newly discovered class of porphyrin isomers. J. Org. Chem. 2014, 79, 4078–4093. [Google Scholar] [CrossRef]




































© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, B.F.O.; Lopes, S.M.M.; Pineiro, M.; Pinho e Melo, T.M.V.D. Current Advances in the Synthesis of Valuable Dipyrromethane Scaffolds: Classic and New Methods. Molecules 2019, 24, 4348. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24234348
Nascimento BFO, Lopes SMM, Pineiro M, Pinho e Melo TMVD. Current Advances in the Synthesis of Valuable Dipyrromethane Scaffolds: Classic and New Methods. Molecules. 2019; 24(23):4348. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24234348
Chicago/Turabian StyleNascimento, Bruno F. O., Susana M. M. Lopes, Marta Pineiro, and Teresa M. V. D. Pinho e Melo. 2019. "Current Advances in the Synthesis of Valuable Dipyrromethane Scaffolds: Classic and New Methods" Molecules 24, no. 23: 4348. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24234348