Co-Existence of Hypertensive and Anti-Hypertensive Constituents, Synephrine, and Nobiletin in Citrus unshiu Peel
Abstract
:1. Introduction
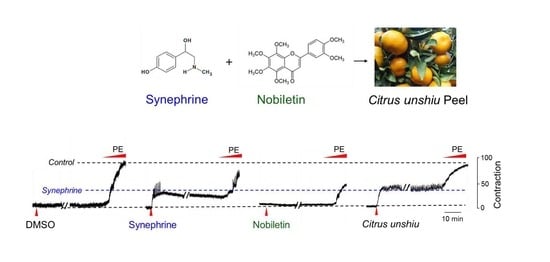
2. Results and Discussion
3. Materials and Methods
3.1. Reagents
3.2. Extraction and Isolation
3.3. Animals
3.4. Measurement of Vasoconstriction in Isolated Aortic Rings
3.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Raskin, I.; Ribnicky, D.M.; Komarnytsky, S.; Ilic, N.; Poulev, A.; Borisjuk, N.; Brinker, A.; Moreno, D.A.; Ripoll, C.; Yakoby, N.; et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002, 20, 522–531. [Google Scholar] [PubMed]
- González-Minero, F.; Bravo-Díaz, L. The use of plants in skin-car products, cosmetics and fragrances: Past and present. Cosmetics 2018, 5, 50. [Google Scholar]
- Aburjai, T.; Natsheh, F.M. Plant used in cosmetics. Phytother. Res. 2003, 17, 987–1000. [Google Scholar] [PubMed]
- Thakur, J.P.; Gothwal, P.P. Edible plants as a source of antitubercular agents. J. Pharmacogn. Phytochem. 2015, 4, 228–234. [Google Scholar]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar]
- Weidner, C.; de Groot, J.C.; Prasad, A.; Freiwald, A.; Quedenau, C.; Kliem, M.; Witzke, A.; Kodelja, V.; Han, C.T.; Giegold, S.; et al. Amorfrutins are potent antidiabetic dietary natural products. Proc. Natl. Acad. Sci. USA 2012, 109, 7257–7262. [Google Scholar] [PubMed] [Green Version]
- Whitebread, S.; Hamon, J.; Bojanic, D.; Urban, L. Keynote review: In vitro safety pharmacology profiling: An essential tool for successful drug development. Drug Discov. Today 2005, 10, 1421–1433. [Google Scholar]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar]
- Wagner, H. Synergy research: Approaching a new generation of phytopharmaceuticals. Fitoterapia 2011, 82, 34–37. [Google Scholar]
- Park, H.-J.; Jung, U.J.; Cho, S.-J.; Jung, H.-K.; Shim, S.; Choi, M.-S. Citrus unshiu peel extract ameliorates hyperglycemia and hepatic steatosis by altering inflammation and hepatic glucose-and lipid-regulating enzymes in db/db mice. J. Nutr. Biochem. 2013, 24, 419–427. [Google Scholar] [PubMed]
- Blumenthal, M. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines; American Botanical Council: Austin, TX, USA, 1998. [Google Scholar]
- Matsubara, Y.; Kumamoto, H.; Iizuka, Y.; Murakami, T.; Okamoto, K.; Miyake, H.; Yokoi, K. Structure and hypotensive effect of flavonoid glycosides in Citrus unshiu peelings. Agric. Biol. Chem. 1985, 49, 909–914. [Google Scholar]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.-S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar]
- Ma, Y.-Q.; Ye, X.-Q.; Fang, Z.-X.; Chen, J.-C.; Xu, G.-H.; Liu, D.-H. Phenolic compounds and antioxidant activity of extracts from ultrasonic treatment of Satsuma mandarin (Citrus unshiu Marc.) peels. J. Agric. Food Chem. 2008, 56, 5682–5690. [Google Scholar]
- Oh, Y.-C.; Cho, W.-K.; Jeong, Y.H.; Im, G.Y.; Yang, M.C.; Hwang, Y.-H.; Ma, J.Y. Anti-inflammatory effect of Citrus Unshiu peel in LPS-stimulated RAW 264.7 macrophage cells. Am. J. Chin. Med. 2012, 40, 611–629. [Google Scholar] [PubMed]
- Noh, H.J.; Hwang, D.; Lee, E.S.; Hyun, J.W.; Yi, P.H.; Kim, G.S.; Lee, S.E.; Pang, C.; Park, Y.J.; Chung, K.H. Anti-inflammatory activity of a new cyclic peptide, citrusin XI, isolated from the fruits of Citrus unshiu. J. Ethnopharmacol. 2015, 163, 106–112. [Google Scholar] [PubMed]
- Choi, H.-S. Lipolytic effects of citrus peel oils and their components. J. Agric. Food Chem. 2006, 54, 3254–3258. [Google Scholar]
- Takayanagi, K.; Morimoto, S.-i.; Shirakura, Y.; Mukai, K.; Sugiyama, T.; Tokuji, Y.; Ohnishi, M. Mechanism of visceral fat reduction in Tsumura Suzuki obese, diabetes (TSOD) mice orally administered β-cryptoxanthin from Satsuma mandarin oranges (Citrus unshiu Marc). J. Agric. Food Chem. 2011, 59, 12342–12351. [Google Scholar]
- Egan, B.; Panis, R.; Hinderliter, A.; Schork, N.; Julius, S. Mechanism of increased alpha adrenergic vasoconstriction in human essential hypertension. J. Clin. Invest. 1987, 80, 812. [Google Scholar] [PubMed]
- Cohn, J.N. Vascular wall function as a risk marker for cardiovascular disease. J. Hypertens. Suppl. 1999, 17, S41–S44. [Google Scholar] [PubMed]
- Centeno, J.M.; Burguete, M.C.; Castelló-Ruiz, M.; Enrique, M.; Vallés, S.; Salom, J.B.; Torregrosa, G.; Marcos, J.F.; Alborch, E.; Manzanares, P. Lactoferricin-related peptides with inhibitory effects on ACE-dependent vasoconstriction. J. Agric. Food Chem. 2006, 54, 5323–5329. [Google Scholar]
- Stohs, S.J. Assessment of the adverse event reports associated with Citrus aurantium (Bitter orange) from April 2004 to October 2009. J. Funct. Foods 2010, 2, 235–238. [Google Scholar]
- Hibino, T.; Yuzurihara, M.; Kase, Y.; Takeda, A. Synephrine, a component of Evodiae fructus, constricts isolated rat aorta via adrenergic and serotonergic receptors. J. Pharmacol. Sci. 2009, 111, 73–81. [Google Scholar] [PubMed]
- Suzuki, M.; Sasaki, K.; Yoshizaki, F.; Fujisawa, M.; Oguchi, K.; Cyong, J.-C. Anti-hepatitis C virus effect of citrus unshiu peel and its active ingredient nobiletin. Am. J. Chin. Med. 2005, 33, 87–94. [Google Scholar] [PubMed]
- Rikako, S.; Hiroyuki, K.; Shigeyuki, S.; Akira, M.; Masamichi, Y.; Hajime, O.; Takuji, T. Citrus Flavonoid Nobiletin Suppresses Azoxymethane-Induced Rat Colon Tumorigenesis. In Potential Health Benefits of Citrus; Patil, B.S., Turner, N.D., Miller, E.G., Brodbelt, J.S.., Eds, *!!! REPLACE !!!*, Eds.; American Chemical Society: Washington, DC, USA, 2006; Volume 936, pp. 104–120. [Google Scholar]
- Ikemura, M.; Sasaki, Y.; Giddings, J.C.; Yamamoto, J. Protective Effects of Nobiletin on Hypertension and Cerebral Thrombosis in Stroke-Prone Spontaneously Hypertensive Rats (SHRSP). Food Nutr. Sci. 2015, 3, 1539–1549. [Google Scholar]
- Lee, Y.-S.; Cha, B.-Y.; Saito, K.; Yamakawa, H.; Choi, S.-S.; Yamaguchi, K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.-T. Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochem. Pharmacol. 2010, 79, 1674–1683. [Google Scholar]
- Lo, Y.H.; Pan, M.H.; Li, S.; Yen, J.H.; Kou, M.C.; Ho, C.T.; Wu, M.J. Nobiletin metabolite, 3′,4′-dihydroxy-5,6,7,8-tetramethoxyflavone, inhibits LDL oxidation and down-regulates scavenger receptor expression and activity in THP-1 cells. Biochim. Biophys. Acta 2010, 1801, 114–126. [Google Scholar] [PubMed]
- Zhou, C.-H.; Wu, X.-H.; Wu, Y.-Q. Nobiletin, a dietary phytochemical, inhibits vascular smooth muscle cells proliferation via calcium-mediated c-Jun N-terminal kinases pathway. Eur. J. Pharmacol. 2009, 615, 55–60. [Google Scholar]
- Takese, H.; Yamamoto, K.; Harano, H.; Saito, Y.; Yamashita, A. Pharmacological profile of gastric mucosal protection by marmin and nobiletin from a traditional herbal medicine, Aurantii fructus immaturus. Jpn. J. Pharmacol. 1994, 66, 139–147. [Google Scholar]
- Xiong, Y.-J.; Chen, D.-P.; Lv, B.-C.; Liu, F.-F.; Wang, L.; Lin, Y. Characteristics of nobiletin-induced effects on jejunal contractility. Fitoterapia. 2014, 94, 1–9. [Google Scholar] [PubMed]
- Yang, W.; Li, S.; Liao, L.; Zheng, X.; Li, J.; Zheng, Y.; Zhang, X.; Zhu, D. Nobiletin relaxes isolated mesenteric arteries by activating the endothelial Ca2+-eNOS pathway in rats. J. Vasc. Res. 2016, 53, 330–339. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-J.; Kim, K.; Jung, Y.-R.; Bian, Y.; Ngo, T.; Bae, O.-N.; Lim, K.-M.; Chung, J.-H. Co-Existence of Hypertensive and Anti-Hypertensive Constituents, Synephrine, and Nobiletin in Citrus unshiu Peel. Molecules 2019, 24, 1197. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24071197
Kim J-J, Kim K, Jung Y-R, Bian Y, Ngo T, Bae O-N, Lim K-M, Chung J-H. Co-Existence of Hypertensive and Anti-Hypertensive Constituents, Synephrine, and Nobiletin in Citrus unshiu Peel. Molecules. 2019; 24(7):1197. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24071197
Chicago/Turabian StyleKim, Jung-Joon, Keunyoung Kim, Ye-Ryeon Jung, Yiying Bian, Thien Ngo, Ok-Nam Bae, Kyung-Min Lim, and Jin-Ho Chung. 2019. "Co-Existence of Hypertensive and Anti-Hypertensive Constituents, Synephrine, and Nobiletin in Citrus unshiu Peel" Molecules 24, no. 7: 1197. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24071197