3.2.1. Procedure for Synthesis of Precursors 1a and 1b (Scheme 2)
(S)-Methyl ((S)-2-((tert-butoxycarbonyl)amino)-3-hydroxypropanamido)-3-phenylpropanoate (
S3) [
26]. To a suspension of Boc-L-Ser (
S1) (1.00 g, 4.9 mmol, 1.0 eq) in CH
2Cl
2 (50 mL, 0.1 M),
N-methylmorpholine was added (1.6 mL, 14.6 mmol, 3.0 eq), followed by the addition of
l-Phe-OCH
3 hydrochloride (
S2) (1.05 g, 4.9 mmol, 1.0 eq). After 0.5 h, the solution was cooled to 0 °C and HOBt (658 mg, 4.9 mmol, 1.0 eq) and EDC (1.02 g, 5.4 mmol, 1 eq) were added and the reaction mixture was stirred for 18 h at room temperature. A solution was dissolved in ethyl acetate (100 mL) and extracted with 10% citric acid (2 × 20 mL), saturated solution NaHCO
3 (2 × 20 mL), brine (20 mL) and dried over anhydrous Na
2SO
4. Organic solvent was evaporated under reduced pressure and the crude product was purified by flash column chromatography in Hex/EtOAc = 1/2 as eluent. White solid (446 mg, 1.2 mmol, 25% yield) [
26]; R
f (Hexane/ethyl acetate = 1/2) = 0.25; mp = 92–93 °C;
1H-NMR (400 MHz, DMSO-
d6):
δ 8.14 (d,
J = 7.8 Hz, 1H), 7.31–7.14 (m, 5H), 6.65 (d,
J = 8.3 Hz, 1H), 4.80 (t,
J = 5.8 Hz, 1H), 4.49 (ddd,
J = 8.0, 7.8, 5.8 Hz, 1H), 3.99 (ddd,
J = 8.3, 6.8, 4.7 Hz, 1H), 3.58 (s, 3H), 3.50, 3.43 (ABXY,
J = 11.2, 5.8, 4.7, 6.8 Hz, 2H), 3.01, 2.94 (ABX, dd,
J =13.5, 8.0, 5.8 Hz, 2H) ppm;
13C-NMR (100 MHz, CDCl
3):
δ 172.0, 171.2, 135.9, 129.4, 128.8, 127.4, 80.6, 63.1, 55.2, 53.6, 52.7, 37.9, 28.4 ppm; MS (ESI
+):
m/
z 389 [M + Na]
+, 289 (100); HRMS (ESI
−): Calc mass for C
18H
25N
2O
6 [M − H]
−: 365.1713; measured: 365.1721; IR (ATR): 3521.6, 3338.0, 2977.8, 1736.1, 1668.1, 1649.2, 1540.3, 1519.9, 1307.3, 1266.4, 1223.9, 1008.3, 698.4, 616.3 cm
-1;
= +33.7 (0.56, MeOH).
(S)-Methyl 2-((S)-2-amino-3-hydroxypropanamido)-3-phenylpropanoate hydrochloride (
S4) [
26]. Compound
S4 was synthesized according to the literature procedure [
26]. To a solution of
S3 (446 mg, 1.2 mmol) in CH
2Cl
2 (5 mL) was added CF
3COOH (1 mL) dropwise at 0 °C, and the solution was stirred at rt for 5 h. Solvent was evaporated and the white residue was dissolved in diethyl ether (10 mL) to which 2 M HCl in diethyl ether (3.0 mL, 6.1 mmol, 5 eq) was added and filtered to afford the product
S4. White needles (306 mg, 1.0 mmol, 83% yield); mp = 149–150 °C;
1H-NMR (400 MHz, MeOD):
δ 7.36–7.19 (m, 5H), 4.75 (dd,
J = 8.7, 5.5 Hz, 1H), 4.04–3.87 (m, 2H), 3.79 (dd,
J = 10.3, 5.9 Hz, 1H), 3.73 (s, 3H), 3.23 (dd,
J = 14.2, 5.4 Hz, 1H), 3.04 (dd,
J = 14.2, 8.7 Hz, 1H) ppm;
13C-NMR (100 MHz, MeOD):
δ 173.1, 168.4, 138.1, 130.4, 129.7, 128.2, 61.8, 56.3, 55.7, 53.0, 38.3 ppm; MS (ESI
+):
m/
z 289 ([M + Na]
+, 100), 267 ([M + H]
+, 80); IR (ATR): 3325.0, 3245.5, 1731.4, 1663.4, 1535.3, 1445.8, 1363.2, 1225.6, 1049.1, 699.2, 617.6 cm
−1;
= +86.0 (0.233, MeOH).
(S)-Methyl ((S)-2-(3-(1,3-dioxoisoindolin-2-yl)-2-oxoazetidin-1-yl)-3-phenylpropanoate (
1a,
Scheme 2). To a solution of
S4 (1.1 g, 3.63 mmol, 1 eq) in DMF (20 mL) phthalanhydride (0.59 g, 3.99 mmol, 1.1 eq) and triethylamine (0.53 mL, 3.81 mmol, 1.05 eq) were added, and the solution was stirred at 100 °C for 18 h. After the mixture was cooled to room temperature, DMF was evaporated under reduced pressure and the oily residue was dissolved in EtOAc (50 mL). Organic phase was washed with water (50 mL), 1 M HCl (50 mL), saturated solution NaHCO
3 (50 mL) and brine (50 mL) and dried over anhydrous Na
2SO
4. Organic solvent was evaporated under reduced pressure yielding a white solid. White solid (1.14 g, 2.9 mmol, 80% yield); R
f (hexane/ethyl acetate = 1/1) = 0.11; mp = 93–96 °C;
1H-NMR (400 MHz, CDCl
3): δ 7.85 (dd,
J = 5.5, 3.1 Hz, 2H), 7.73 (dd,
J = 5.5, 3.1 Hz, 2H), 7.33–7.37 (m, 2H), 7.27–7.30 (m, 1H), 7.22–7.24 (m, 2H), 5.25 (dd,
J = 5.6, 3.0 Hz, 1H,), 4.87 (dd,
J = 9.3, 6.2 Hz, 1H), 3.90 (dd,
J = 5.4, 3.0 Hz, 1H), 3.83 (s, 3H), 3.64 (t,
J = 5.6 Hz, 1H), 3.29 (dd,
J = 14.3, 6.2 Hz, 1H), 3.08 (dd,
J = 14.3, 9.3 Hz, 1H) ppm;
13C-NMR (100 MHz, CDCl
3): 135.8, 134.4, 131.7, 128.9, 128.7, 127.7, 123.3, 55.0, 53.1, 52.6, 45.2, 35.8 ppm; MS (ESI
+):
m/
z 419 [M + Na, 100]
+; HRMS (ESI
+): Calc mass for C
21H
21O
6N
2: 397.1394 [M + H]
+; measured: 397.1406; IR (ATR): 1711.4, 1675.8, 1529.2, 1467.8, 1437.5, 1386.1, 1215.2, 1115.5, 1079.7, 1030.3, 983.2, 911.3, 877.3, 789.1, 719.7, 702.4, 648.9, 531.4, 504.7 cm
−1;
= −213.0 (0.424, MeOH).
Methyl trityl-l-seryl-l-phenylalaninate (
1b, Scheme 2). To a solution of dipeptide
S4 (500 mg, 1.7 mmol, 1.0 eq) in chloroform (5.5 mL, 0.3 M), trimethylamine (690 μL, 5.0 mmol, 3 eq) was added. After 5 min, the solution was cooled to 0 °C followed by the slow addition of trityl chloride (576 mg, 2.0 mmol, 1.2 eq) at 0 °C. The reaction mixture was stirred for 18 h at room temperature. Ethyl acetate (50 mL) was added and the mixture was extracted with 10% citric acid (2 × 10 mL), saturated solution NaHCO
3 (2 × 10 mL), brine (10 mL) and dried over anhydrous Na
2SO
4. Organic solvent was evaporated under reduced pressure and the crude product was recrystallized from diethyl ether. White crystals (220 mg, 432 mmol, 26% yield); R
f (hexane/ethyl acetate = 1/1) = 0.29; mp = 118–120 °C;
1H-NMR (400 MHz, CDCl
3): δ 7.79 (d,
J = 8.5 Hz, 1H), 7.47–7.12 (m, 20H), 4.85 (ddd,
J = 8.5, 6.3, 6.0 Hz, 1H), 3.79 (s, 3H), 3.48, 2.32 (AMXY,
J = 11.1, 5.5, 2.8, 5.7 Hz, 2H), 3.21 (bs, 1H), 3.16, 3.13 (ABX,
J = 14.6, 6.0, 6.3 Hz, 2H), 2.80 (bs, 1H), 1.67 (t,
J = 5.5 Hz, 1H) ppm;
13C-NMR (100 MHz, CDCl
3):
δ 173.7, 172.2, 146.0, 136.1, 129.5, 129.0, 128.8, 128.3, 127.5, 126.9, 71.7, 63.4, 59.1, 53.0, 52.8, 38.1 ppm; MS (ESI
+):
m/
z 531 ([M + Na]
+, 100); HRMS (ESI
−): Calc mass for C
32H
31N
2O
4 ([M − H]
−, 100): 507.2284; measured: 507.2277; IR (ATR): 3593.4, 3468.1, 332.0, 2948.7, 1739.8, 1668.0, 1491.1, 1198.9, 1016.0, 743.6, 706.1 cm
−1;
= +198.4 (0.310, MeOH).
3.2.4. General Procedure for the Synthesis of 2, 3a, 3c via Mitsunobu Reaction (Scheme 2 and Scheme 3)
To a solution of dipeptide 1a or 1c (0.2 mmol, 1.0 eq) and PPh3 (1.5 eq) in anhydrous tetrahydrofuran DIAD (1.5 eq) was added drop-wise at 0 °C under argon atmosphere. After the solution was stirred for 18 h at room temperature the solvent was evaporated and the crude product was purified by flash chromatography.
Methyl (2-(1,3-dioxoisoindolin-2-yl)acryloyl)-l-phenylalaninate (
2,
Scheme 2). White solid (20 mg, 0.052 mmol, 21% yield; R
f (Hexane/ethyl acetate = 1/2) = 0.51; mp = 63–65 °C;
1H-NMR (400 MHz, CDCl
3): δ 7.91 (dd,
J = 5.5, 3.1 Hz, 2H), 7.78 (dd,
J = 5.5, 3.1 Hz, 2H), 7.21–7.30 (m, 3H), 7.15–7.17 (m, 5H), 6.48 (d,
J = 7.6 Hz, 1H), 6.08 (d,
J = 1,4 Hz, 1H), 5.82 (d,
J = 1,4 Hz, 1H), 4.96 (dt,
J = 7.6, 5.3 Hz, 1H), 3.76 (s, 3H), 3.22 (t,
J = 5.2 Hz, 2H) ppm;
13C-NMR (100 MHz, CDCl
3):
δ 171.6, 166.5, 162.6, 135.7, 134.7, 132.7, 131.8, 128.7, 128.6, 127.3, 124.1, 120.5, 53.7, 52.6, 37.7 ppm; MS (ESI
+):
m/
z 401 [M + Na]
+; MS (ESI
−):
m/
z 377 [M − H]
+; HRMS (ESI
+): Calc mass for C
21H
19O
5N
2 [M + H]
+ 379.1288; measured: 379.1287; IR (ATR): 3299.1, 1789.4, 1722.6, 1656.8, 1624.7, 1536.3, 1454.7, 1378.1, 1303.2, 1273.6, 1207.7, 1172.2, 1116.3, 1083.1, 1031.3, 995.4, 950.6, 920.1, 883.4, 788.1, 735.2, 701.3, 630.1, 572.4, 532.5 cm
−1;
= −585,9 (0.375, MeOH).
Methyl (S)-2-((S)-3-(1,3-dioxoisoindolin-2-yl)-2-oxoazetidin-1-yl)-3-phenylpropanoate (
3a,
Scheme 2). White solid (14 mg, 0.037 mmol, 15% yield); R
f (chloroform/acetone = 100/5) = 0.39; mp =122–124 °C;
1H-NMR (400 MHz, CDCl
3):
δ 7.88 (dd,
J = 5.5, 3.0 Hz, 2H), 7.77 (dd,
J = 5.5, 3.0 Hz, 2H), 7.16–7.20 (m, 3H), 7.05–7.07 (m, 2H), 6.80 (d,
J = 7.7 Hz, 1H), 4.86–4.91 (m, 2H), 4.40 (dd,
J = 11.5, 6.8 Hz, 1H), 4.03 (dd,
J= 11.5, 5.3 Hz, 1H), 3.73 (s, 3H), 3.13 (dd,
J = 5.7, 4.1 Hz, 2H) ppm;
13C-NMR (100 MHz, CDCl
3): δ 171.7, 168.0, 167.4, 135.6, 134.5, 131.7, 129.3, 128.6, 127.2, 123.7, 60.9, 54.5, 53.51, 52.5, 37.7 ppm; MS (ESI
−):
m/
z 376.5 [M − H]
−; MS (ESI
+)
m/
z 400.6 [M + Na]
+; HRMS (ESI
+): Calc mass for C
21H
19O
5N
2 [M + H]
+: 379.1288; measured: 379.1298; IR (ATR): 1763.4, 1740.4, 1717.2, 1391.3, 1205.2, 1115.8, 1025.9, 716.9, 701.6 cm
−1;
= −507.8 (0.546, MeOH).
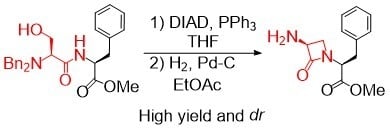
Methyl (S)-2-((S)-3-(dibenzylamino)-2-oxoazetidin-1-yl)-3-phenylpropanoate (
3cS,
Scheme 2). Yellow oil (76% yield); R
f (hexane/ethyl acetate = 6/1) = 0.19;
1H-NMR (400 MHz, CDCl
3):
δ 7.39–7.11 (m, 16H), 4.70 (dd,
J = 10.0, 5.6 Hz, 1H), 4.13 (dd,
J = 5.2, 2.5 Hz, 1H), 3.74, 3.64 (AB,
J = 13.7 Hz, 4H), 3.39, 3.18 (AMX,
J = 5.6, 5.2, 2.5 Hz, 2H), 3.71 (s, 3H), 3.22, 3.00 (ABX,
J = 13.4, 10.0, 5.6 Hz, 2H) ppm;
13C-NMR (100 MHz, CDCl
3): δ 170.8, 169.7, 138.6, 136.4, 129.1, 128.9, 128.8, 128.5, 127.4, 127.3, 68.5, 54.8, 54.7, 52.7, 43.2, 35.7 ppm; MS (ESI
+):
m/
z 429 [M + H]
+; HRMS (ESI
−): Calc mass for C
27H
29O
3N
2 [M − H]
−: 429.2173; measured: 429.2170; IR (ATR): 3028.4, 2952.4, 1744.9, 1447.4, 1220.3, 744.2, 700.3 cm
−1;
= −34.5 (0.38, MeOH).
Methyl (R)-2-((S)-3-(dibenzylamino)-2-oxoazetidin-1-yl)-3-phenylpropanoate (
3cR,
Scheme 2). Yellow oil (9% yield); R
f (hexane/ethyl acetate = 6/1) = 0.13;
1H-NMR (400 MHz, CDCl
3):
δ 7.38–7.08 (m, 15H), 4.85 (dd,
J = 11.3, 5.1 Hz, 1H), 4.25 (dd,
J = 5.0, 2.4 Hz, 1H), 3.72 (s, 3H), 3.40, 3.24 (AB,
J = 14.0 Hz, 4H), 3.34, 3.11 (AMX,
J = 5.5, 5.0, 2.4 Hz, 1H), 3.31, 2.95 (AMX,
J = 14.6, 5.1, 11.3 Hz 2H) ppm;
13C-NMR (100 MHz, CDCl
3): δ 170.7, 169.5, 138.5, 136.2, 129.0 (2 signals), 128.9, 128.5, 127.4, 127.3, 68.7, 54.4, 53.8, 52.7, 42.7, 36.0 ppm; HRMS (ESI
−): Calc mass for C
27H
29O
3N
2 [M − H]
−: 429.2173; measured: 429.2172; IR (ATR): 3332.8, 3027.8, 2952.5, 1736.2, 1453.9, 1259.3, 1221.4, 1096.2, 1027.9, 735.4, 697.0 cm
−1.
3.2.5. Synthesis of Chlorides 6a and 6b (Scheme 2)
Compounds 6a and 6b were synthesized according to the procedures described for the preparation of 5 using SO2Cl2 or CH3SO2Cl to obtain a crude mixture, which was purified with flash column chromatography. However, a mixture of both compounds 6a and 6b in equimolar ratio was obtained, which was further purified with preparative HPLC. A separation was possible, but the compounds were transformed back upon treatment to give mixture of 6a:6b in a ratio 1:1 again. The reported data are given for a mixture of both compounds. Colorless liquid (60% yield); Rf (hexane/diethyl ether = 3/1) = 0.36; 1H-NMR (400 MHz, acetone-d6): δ 7.84 (d, J = 7.9 Hz, 1H), 7.65 (d, J = 8.0 Hz, 1H), 4.82 (ddd, J = 8.8, 8.0, 5.1 Hz, 1H), 4.72 (ddd, J = 8.1, 7.9, 5.6 Hz, 1H), 4.47 (dd, J = 6.9, 6.4 Hz, 1H), 4.00, 3.88 (ABX, J = 11.3, 6.8, 6.7 Hz, 2H), 3.75, 3.53 (AX, J = 13.9 Hz, 4H), 3.71, 3.58 (AB, J = 13.7 Hz, 4H), 3.50 (dd, J = 6.7, 6.8 Hz, 1H), 3.28, 3.08 (AMX, J = 14.0, 8.8, 5.1 Hz, 2H), 3.18, 3.07 (ABX, J = 13.9, 5.6, 8.1 Hz, 2H), 3.12, 2.83 (AMX, J = 13.5, 6.9, 6.4 Hz, 2H,) ppm; 13C-NMR (100 MHz, acetone-d6): δ 172.6, 172.2, 170.0, 168.6, 140.1, 139.8, 138.0, 137.8, 130.3, 129.9, 129.7, 129.4, 129.3, 129.2, 129.1, 128.0, 127.7, 64.6, 58.9, 58.3, 56.6, 55.1, 55.0, 54.4, 52.3, 52.5, 42.3, 38.2, 38.0 ppm; MS (ESI+): m/z 429 [M + H]+; HRMS (ESI+): Calc mass for C27H29ClNO3 [M + H]+: 465.1939; measured: 465.1928; IR (ATR): 3028.6, 1737.1, 1603.2, 1494.4, 1453.1, 1377.9, 1256.4, 1221.8, 1174.0, 1128.7, 1026.1, 915.4, 745.9, 698.7 cm−1
3.2.6. Synthesis of Tyrosine Derivatives 1d–1f (Scheme 3)
Methyl (R)-3-(4-(benzyloxy)phenyl)-2-((tert-butoxycarbonyl)amino)propanoate (
S8a) [
29]. Benzyl protected compound
S8a was synthesized according to the modified literature procedure [
29] from methyl (
tert-butoxycarbonyl)-
d-tyrosinate (
S7) (8.80 g, 29.8 mmol, 1 eq) which was dissolved in acetone (160 mL). Potassium carbonate (4.12 g, 29.8 mmol, 1 eq) was added and the mixture obtained was cooled to 0 °C followed by dropwise addition of benzyl bromide (3.9 mL, 32.9 mmol, 1.1 eq) and the reaction mixture was stirred for 24 h at room temperature. Ethyl acetate (200 mL) was then added and the solution was washed with water (100 mL). Water layer was then extracted with ethyl acetate (2 × 50 mL) and the combined organic fractions were washed with brine, dried over anhydrous sodium sulfate, filtered and evaporated. A crude mixture was purified with flash column chromatography using hexane/ethyl acetate = 3:1 as eluent. White amorphous solid (10.77 g, 27.9 mmol, 94% yield); mp = 83–86 °C; R
f (hexane:ethyl acetate = 3:1) = 0.38;
1H-NMR (400 MHz, CDCl
3):
δ 7.45–7.28 (m, 5H), 7.03 (d,
J = 8.6 Hz, 2H), 6.90 (d,
J = 8.6 Hz, 2H), 5.03 (s, 2H), 4.98 (d,
J = 8.0 Hz, 1H), 4.54 (ddd,
J = 8.0, 6.1, 5.7 Hz, 1H), 3.70 (s, 3H), 3.05, 2.99 (ABX,
J = 14.1, 6.1, 5.7 Hz, 2H), 1.42 (s, 9H) ppm;
13C-NMR (100 MHz, CDCl
3):
δ 172.5, 157.9, 155.2, 137.1, 130.4, 128.6, 128.3, 128.0, 127.5, 114.9, 79.9, 70.0, 54.6, 52.2, 37.4, 28.4 ppm; MS (ESI
+): 408 [M + Na]
+; IR (ATR): 3367.2, 2977.4, 1742.6, 1710.4, 1509.8, 1365.4, 1241.0, 1161.1, 1016.9, 732.9 cm
−1;
= −4.3 (0.66, MeOH).
Methyl (R)-3-(4-acetoxyphenyl)-2-((tert-butoxycarbonyl)amino)propanoate (
S8b)
[30]. Acetyl protected compound
S8b was synthesized from methyl (
tert-butoxycarbonyl)-
d-tyrosinate (
S7) (8.02 g, 27.2 mmol, 1 eq) which was dissolved in pyridine (150 mL) and the mixture obtained was cooled to 0 °C followed by dropwise addition of acetanhydride (12.7 mL, 136 mmol, 5 eq) and the reaction mixture was stirred for 24 h at room temperature. Ethyl acetate (200 mL) was then added and the solution was washed with 1 M HCl (2 × 50 mL), saturated NaHCO
3 (2 × 50 mL) and brine (50 mL). Organic fraction was dried over anhydrous sodium sulfate, filtered and evaporated. A crude mixture was purified by crystallization from diethyl ether. White amorphous solid (8.65 g, 25.5 mmol, 94% yield); mp = 54–56 °C;
1H-NMR (400 MHz, DMSO-
d6):
δ 7.26 (d,
J = 8.4 Hz, 2H), 7.03 (d,
J = 8.4 Hz, 2H), 4.17 (ddd,
J = 10.2, 8.1, 5.0 Hz, 1H), 3.61 (s, 3H), 2.99, 2.85 (ABX,
J = 13.8, 10.4, 5.0 Hz, 2H), 2.25 (s, 3H), 1.32 (s, 9H) ppm;
13C-NMR (100 MHz, CDCl
3):
δ 173.0, 169.7, 155.9, 149.6, 130.5, 122.0, 78.8, 55.6, 52.3, 36.2, 28.6, 21.3 ppm; MS (ESI
+): 359.9 [M + Na]
+; IR (ATR): 3375.4, 2980.2, 1748.4, 1684.6, 1502.1, 1368.3, 1216.5, 1163.9, 1022.4 cm
−1;
= +5.3 (0.56, 8.5 mg CH
3CN).
Methyl (R)-2-(4-(benzyloxy)phenyl)-2-((tert-butoxycarbonyl)amino)acetate (
S8c) [
31]. Benzyl protected compound
S8c was synthesized from methyl (
R)-2-(4-(benzyloxy)phenyl)-2-((
tert-butoxycarbonyl) amino)acetate (
S7c) by the literature described procedure [
31]. Yelow oil (8.735 g, 23.5 mmol, 98% yield); R
f (Hexane/ethyl acetate = 1/1) = 0.64;
1H-NMR (400 MHz, CDCl
3) δ 1.43 (s, 9H), 3.71 (s, 3H), 5.05 (s, 2H), 5.25 (d,
J = 7,1 Hz, 1H), 6.95 (d,
J = 8.7 Hz, 2H), 7.27 (d,
J = 8.7 Hz, 2H), 7.33−7.43 (m, 6H) ppm.
(R)-3-(4-(Benzyloxy)phenyl)-1-methoxy-1-oxopropan-2-aminium trifluoroacetate (
S9a) [
31]. Amine
S9a was synthesized according to the modified literature procedure for
S enantiomer [
32] from
S8a (10.6 g, 27.5 mmol, 1 eq), which was dissolved in CH
2Cl
2 (90 mL). Trifluoroacetic acid (8.4 mL, 110 mmol, 4 eq) was added and after 18 h of stirring overnight, the solution was evaporated and crystalized from diethyl ether. White amorphous solid (9.58 g, 24.0 mmol, 87% yield); mp = 117–119 °C;
1H-NMR (400 MHz, DMSO-
d6):
δ 8.45 (s, 3H), 7.47–7.30 (m, 5H), 7.14 (d,
J = 8.6 Hz, 2H), 6.99 (d,
J = 8.6 Hz, 2H), 5.09 (s, 2H), 4.54 (t,
J = 6.5 Hz, 1H), 3.69 (s, 3H), 3.01, 3.06 (ABX,
J = 14.2, 6.5 Hz, 2H) ppm; MS (ESI
+): 286 [M + H]
+; IR (ATR): 3034.0, 1742.1, 1662.6, 1610.9, 1513.1, 1449.0, 1243.1, 1179.3, 1135.1, 836.8, 721.2 cm
−1;
= −15.4 (0.45, MeOH).
(R)-3-(4-Acetoxyphenyl)-1-methoxy-1-oxopropan-2-aminium trifluoroacetate (
S9b) [
30]. Amine
S9b was synthesized according to the modified literature procedure for
S enantiomer [
30] from
S8b (10.6 g, 27.5 mmol, 1 eq), which was dissolved in CH
2Cl
2 (90 mL). Trifluoroacetic acid (8.4 mL, 110 mmol, 4 eq) was added and after 18 h of stirring overnight, the solution was evaporated and used in further reactions without purification.
(R)-1-(4-(Benzyloxy)phenyl)-2-methoxy-2-oxoethan-1-aminium chloride (
S9c) [
33]. Amine
S9c was synthesized according to the modified literature procedure [
33] from
S8c. White powder (6.869 g, 23.5 mmol, 93% yield); R
f (Hexane/ethyl acetate = 1/1) = 0.07;
1H-NMR (400 MHz, DMSO-
d6):
δ 3.38 (s, 3H), 3.70 (s, 3H), 5.14 (s, 2H), 5.20 (s, 1H), 7.08 (d,
J = 8.8 Hz, 2H), 7.27−7.45 (m, 7H) ppm.
Methyl (R)-3-(4-(benzyloxy)phenyl)-2-((S)-2-(dibenzylamino)-3-hydroxypropanamido) propanoate (
1d,
Scheme 3). Dipeptide methyl dibenzyl-
l-seryl-
l-phenylalaninate (
1d) was synthesized in a coupling procedure similar to the synthesis of
S3 starting from dibenzyl-
l-serine (
S6) (7.02 g, 24.6 mmol, 1.05 eq) and
S9 (9.33 g, 23.4 mmol, 1.00 eq). Yellow liquid (7.53 g, 77% yield); R
f (Hexane:ethyl acetate = 1:2) = 0.45;
1H-NMR (400 MHz, CDCl
3):
δ 7.74 (d,
J = 8.9 Hz, 1H), 7.49–7.42 (m, 2H), 7.41–7.34 (m, 2H), 7.34–7.20 (m, 7H), 7.19–7.11 (m, 4H), 6.99–6.90 (m, 4H), 5.08, 5.04 (AB,
J = 11.8 Hz, 2H), 4.85 (ddd,
J = 8.9, 5.3, 5.5 Hz, 1H), 4.11, 3.97 (ABX,
J = 11.4, 7.5, 4.0 Hz, 2H), 3.84, 3.43 (AB,
J = 13.3 Hz, 4H), 3.62 (s, 3H), 3.30 (dd,
J = 7.5, 4.0 Hz, 1H), 3.12, 2.98 (ABX,
J = 13.7, 5.6, 5.2 Hz, 2H) ppm;
13C-NMR (100 MHz, CDCl
3):
δ 174.1, 171.8, 158.3, 138.5, 136.9, 130.5, 128.9, 128.8, 128.6, 128.2, 127.8, 127.6 (2 peaks), 115.2, 70.1, 61.7, 57.4, 54.7,52.6, 52.4, 36.8 ppm; MS (ESI
+):
m/
z 575 [M + Na]
+; MS (ESI
−):
m/
z 551 [M − H]
+; HRMS (ESI
−): Calc mass for C
34H
35N
2O
5 [M − H]
−: 551.2546; measured: 551.2542; IR (ATR): 3367.6, 3029.7, 1741.7, 1662.7, 1509.0, 1241.3, 1176.2, 1026.8, 909.0, 729.8, 696.3 cm
−1;
= +17.4 (c 1.0, MeOH).
Methyl (S)-2-(4-(benzyloxy)phenyl)-2-(2-(dibenzylamino)-3-hydroxypropanamido)acetate (
1e,
Scheme 3). Dipeptide methyl (
S)-2-(4-(benzyloxy)phenyl)-2-(2-(dibenzylamino)-3-hydroxypropanamido) acetate (
1e) was synthesized as a mixture of diastereoisomers in a coupling procedure similar to the synthesis of
S3 starting from dibenzyl-
l-serine (
S6) (10.0 g, 35.0 mmol, 1 eq) and
S9c (12.96 g, 42.0 mmol, 1.20 eq). Orange oil (10.368 g, 19.3 mmol, 55% yield); R
f (Hexane/ethyl acetate = 1/1) = 0.31;
1H-NMR (400 MHz, CDCl
3):
δ 8.46 (d,
J = 7.1 Hz, 0.5H), 8.37 (d,
J = 7.4 Hz, 0.5H), 7.27−7.42 (m, 15H), 7.20 (d,
J = 8.7 Hz, 1H), 7.13 (d,
J = 8.7 Hz, 1H), 6.89 (dd,
J = 13.2, 8.7 Hz, 2H), 5.44 (d,
J = 2.2 Hz, 0.5H), 5.42 (d,
J = 2.2 Hz, 0.5H), 5.05 (s, 1H), 5.02 (s, 1H), 4.14−4.17 (m, 0,5H), 4.04−4.08 (m, 0.5H), 3.93−4.03 (m, 2H), 3.84 (d,
J = 13.6 Hz, 1H), 3.75 (s, 1.5H), 3.73 (s, 1.5H), 3.57 (d,
J = 4.1 Hz, 1H), 3.53 (d,
J = 4.5 Hz, 1H), 3.40 (dd,
J = 7.5, 4.0 Hz, 0.5H), 3.34 (dd,
J = 7.5, 3.7 Hz, 0.5H), 3.25−3.31 (m, 1H) ppm;
13C-NMR (100 MHz, MeOD):
δ 173.6; 171.2; 158.9; 138.5; 136.7; 128.9; 128.7; 128.5; 128.4; 128.3; 128.1; 127.6; 127.5; 115.3; 70.1; 62.1; 57.8; 55.7; 54.8; 52.9 ppm; MS (ESI
+): 561 [M + Na]
+; 537.2 [M − H]
−; HRMS (ESI
−): Calc mass for C
33H
33N
2O
5 [M − H]
−: 537.2395; measured: 537.2399; IR (ATR): 3480, 3363, 3032, 2951, 1728, 1666, 1608, 1505, 1453, 1435, 1383, 1302, 1247, 1173, 1138, 1079, 1012, 992, 905, 855, 806, 743, 700, 646, 609, 575, 524, 505 cm
−1.
Methyl (R)-3-(4-acetoxyphenyl)-2-((S)-2-(dibenzylamino)-3-hydroxypropanamido)propanoate (
1f,
Scheme 3). Dipeptide methyl dibenzyl-
l-seryl-
l-phenylalaninate (
1f) was synthesized in a coupling procedure similar to the synthesis of
S3 starting from dibenzyl-
l-serine (
S6) (12.14 g, 24.6 mmol, 1.3 eq) and
S9b (8.50 g, 32.7 mmol, 1.00 eq) [
30]. White crystals (8.32 g, 52% yield); mp =116–117 °C; R
f (Hexane: ethyl acetate = 1:1) = 0.32;
1H-NMR (400 MHz, CDCl
3):
δ 7.76 (d,
J = 8.8 Hz, 1H), 7.35–7.14 (m, 10H), 7.04–6.98 (m, 4H), 4.88 (ddd,
J = 8.8, 5.6, 5.6 Hz, 1H), 4.14, 4.01 (ABX,
J = 11.4, 7.5, 4.6 Hz, 2H), 3.88, 3.51. (AB,
J = 13.4 Hz, 4H), 3.66 (s, 3H), 3.34 (dd,
J = 7.4, 3.9 Hz, 1H), 3.13, 3.09 (ABX,
J = 14.0, 5.8, 5.4 Hz, 2H), 2.35 (s, 3H) ppm;
13C-NMR (100 MHz, CDCl
3):
δ 174.0, 171.6, 169.5, 150.0, 138.5, 133.6, 130.4, 128.9, 128.7, 127.6, 122.0, 61.8, 57.5, 54.7, 52.6, 52.5, 37.3, 21.3 ppm; HRMS (ESI
+): Calc mass for C
29H
33N
2O
6 505.2333 [M + H]
+; measured: 505.2335; IR (ATR): 3460.3, 3328.0, 1758.3, 1738.2, 1669.4, 1669.4, 1503.7, 1215.6, 1194.9, 914.7, 755.4, 701.8, 585.5 cm
−1;
= −99.0 (c 0.546, MeOH).
Methyl (R)-3-(4-(benzyloxy)phenyl)-2-((S)-3-(dibenzylamino)-2-oxoazetidin-1-yl)propanoate (
3d,
Scheme 3). β-Lactam
3d was synthesized from dipeptide
1d (7.37 g, 13.3 mmol) according to the procedure described for the preparation of
3c using PPh
3 and DIAD in THF to obtain a crude mixture, which was purified with flash column chromatography on silica using petroleum ether:acetone = 10:3 as eluent. White crystals (7.06 g, 99% yield); R
f (petroleum ether:acetone = 10:3) = 0.22; mp = 92–93 °C,
1H-NMR (400 MHz, CDCl
3) δ 7.41–7.17 (m, 15H), 7.13 (d,
J = 8.7 Hz, 2H), 6.82 (d,
J = 8.7 Hz, 2H), 4.84 (dd,
J = 11.5, 5.1 Hz, 1H), 4.72, 4.69 (AB,
J = 11.7 Hz, 2H), 4.25 (dd,
J = 5.1, 2.3 Hz, 1H), 3.72 (s, 3H, 3.38, 3.22 (AB,
J = 13.7 Hz, 4H), 3.36, 3.10 (AMX,
J = 5.6, 5.1, 2.3 Hz, 2H), 3.26, 2.89 (AMX,
J = 14.7, 5.1, 11.5 Hz, 2H) ppm;
13C-NMR (100 MHz, CDCl
3): δ 170.7, 169.4, 158.0, 138.4, 136.9, 129.8, 128.9, 128.6, 128.3, 128.2, 128.0, 127.5, 127.2, 115.1, 69.7, 68.7, 54.2, 53.5, 52.5, 42.5, 35.1 ppm; HRMS (ESI
+): Calc mass for C
34H
35N
2O
4: 535.2597; measured: 535.2596 [M + H]
+; IR (ATR): 3443.2, 1743.8, 1665.6, 1496.5, 1195.6, 746.4, 699.5 cm
−1;
= +59.5 (c 0.347, MeOH).
Methyl (R)-2-(4-(benzyloxy)phenyl)-2-(3-(dibenzylamino)-2-oxoazetidin-1-yl)acetate (
3e,
Scheme 3). β-Lactam
3e was synthesized from dipeptide
1e (9.12 g, 34.8 mmol) according to the procedure described for the preparation of
3c using PPh
3 and DIAD in THF to obtain a crude mixture, which was purified with flash column chromatography on silica using hexane/ethyl acetate = 2/1 as eluent to obtain diastereoisomeric mixture of products. Yellow gel (7.30 g, 14.0 mmol, 32% yield), R
f (Hexane/ethyl acetate = 2/1) = 0.26;
1H-NMR (400 MHz, MeOD)
δ 7.17–7.43 (m, 17H), 6.99–7.02 (m, 2H), 5.54 (s, 0.4H), 5.53 (s, 0.6H), 4.31 (dd,
J = 5.0, 2.4 Hz, 0.4H), 4.19 (dd,
J = 5.1, 2.4 Hz, 0.6H), 3.77–3.80 (m, 2H), 3.71–3.72 (m, 3H), 3.63–3.69 (m, 2H), 3.55 (dd,
J = 5.7, 2.3 Hz, 0.6H), 3.51 (d,
J = 13.5 Hz, 2H), 3.46 (t,
J =5.2 Hz, 0.4H), 3.02 (dd,
J = 5.9, 2.4 Hz, 0.4H), 2.92 (t,
J = 5.4 Hz, 0.6H) ppm;
13C-NMR (100 MHz, MeOD):
δ 171.5; 171.1; 160.6; 139.6; 139.4; 138.4; 130.8; 130.2; 130.2; 129.6; 129.4; 129.0; 128.6; 128.4; 128.4; 127.0; 126.6; 116.5; 116.5; 71.1; 69.2; 61.6; 58.5; 58.4; 55.7; 55.5; 53.2; 44.19; 43.8 ppm; MS (ESI
+): 542.7 [M + (Na)]
+; IR (ATR): 3245.3, 3033.1, 2983.6, 1734.7, 1688.2, 1610.7, 1513.3, 1454.1, 1378.2, 1255.5, 1177.6, 1109.4, 1052.1, 1023.3, 925.3, 852.2, 793.7, 743.8, 699.4, 634.2, 597.1 cm
−1; HRMS (ESI
+): Calc mass for C
33H
33N
2O
4 [M + H]
+: 521.2435; measured: 521.2432.
Methyl (R)-3-(4-acetoxyphenyl)-2-((S)-3-(dibenzylamino)-2-oxoazetidin-1-yl)propanoate (
3f,
Scheme 3). β-Lactam
3f was synthesized from dipeptide
1f (91 mg, 180 μmol) according to the procedure described for the preparation of
3c using PPh
3 and DIAD in THF to obtain a crude mixture, which was purified with flash column chromatography on silica using CH
2Cl
2:acetone = 50:1 as eluent. Viscous oil (139 mg, 90% yield); R
f (CH
2Cl
2:acetone = 50:1) = 0.17;
1H-NMR (400 MHz, CDCl
3):
δ 7.34–7.18 (m, 12H), 6.99 (d,
J = 8.5 Hz, 2H), 4.77 (dd,
J = 10.7, 5.4 Hz, 1H), 4.25 (dd,
J = 5.1, 2.4 Hz, 1H), 3.70 (s, 3H), 3.48, 3.34 (AB,
J = 13.4 Hz, 4H), 3.32, 3.10 (AMX,
J = 5.5, 5.1, 2.5 Hz, 2H), 3.27, 2.98 (AMX,
J = 14.7, 10.8, 5.5 Hz, 2H) ppm;
13C-NMR (100 MHz, CDCl
3): δ 170.4, 169.4, 169.3 (2 signals overlapping), 149.8, 138.3, 133.7, 129.8, 129.0, 128.4, 127.2, 121.9, 68.7, 54.5, 54.0, 52.6, 42.9, 35.2 ppm; HRMS (ESI
+): Calc mass for C
29H
31O
5N
2 [M + H]
+: 487.2227, measured: 487.2221; IR (ATR): 2952.4, 1735.1, 1508.6, 1369.8, 1369.8, 1193.5, 1104.6, 1018.3, 748.1, 699.0 cm
−1;
= +61.2 (0.623, MeOH).
Methyl (R)-2-((S)-3-(dibenzylamino)-2-oxoazetidin-1-yl)-3-(4-hydroxyphenyl)propanoate (
8,
Scheme 3). β-Lactam
8 was synthesized from acetate
3f (4.30 g, 8.84 mmol) in MeOH (88 mL) to which a 1 M aqueous solution of LiOH (10.60 mL, 10.6 mmol, 1.2 eq) was added dropwise at 0 °C during 3 h. The solution was neutralized with acetic acid and evaporated. Viscous oil (3.53 g, 90% yield); R
f (CH
2Cl
2:acetone = 30:1) = 0.33;
1H-NMR (400 MHz, CDCl
3):
δ 7.31–7.24 (m, 8H, Ar-H), 7.23–7.17 (m, 2H, Ar-H), 6.99 (d,
J = 8.41 Hz, 2H), 6.66 (d,
J = 8.6 Hz, 2H), 4.78 (dd,
J = 11.1, 5.3 Hz, 1H), 4.26 (dd,
J = 5.0, 2.4 Hz, 1H), 3.69 (s, 3H), 3.45, 3.32 (AB,
J = 13.4 Hz, 4H), 3.36, 3.16 (AMX,
J = 5.5, 5.0, 2.4 Hz, 2H), 3.21, 2.87 (AMX,
J = 14.6, 11.1, 5.3 Hz, 2H) ppm;
13C-NMR (100 MHz, CDCl
3):
δ 170.6, 170.2, 155.6, 138.2, 129.7, 128.8, 128.3, 127.2, 127.0, 115.9, 68.2, 54.3 (2 signals overlapping), 52.6, 43.0, 35.0 ppm; HRMS (ESI
+): Calc mass for C
27H
29O
4N
2: 445.2122, measured: 445.2113 [M + H]
+; IR (ATR): 3308.9, 3027.4, 2953.18, 1724.3, 1516.6, 1221.8, 1173.9, 731.7, 697.7 cm
−1;
= +56.2 (c 0.623, MeOH).
3.2.7. Deprotection of Dibenzylamine from 3c–3f and 8 for the Preparation of 7c–7e (Scheme 3)
Methyl (S)-2-((S)-3-amino-2-oxoazetidin-1-yl)-3-phenylpropanoate (
7c,
Scheme 3). Dibenzylamine
3c (450 mg, 1.05 mmol) was dissolved in a mixture of ethyl acetate and ethanol (95:5) and the solution was flushed with argon. Palladium on activated carbon (5% Pd, 90 mg) was added and the suspension was slowly flushed with hydrogen for 30 min. The mixture was stirred under hydrogen for 24 h, palladium was then removed by filtration through a pad of Celite and the solution obtained evaporated. The crude product was purified with flash column chromatography on silica using CH
2Cl
2:MeOH = 20:1 as eluent. White crystals (156 mg, 0.63 mmol, 60% yield); mp = 274–276 °C; R
f (CH
2Cl
2:MeOH = 20:1) = 0.17;
1H-NMR (400 MHz, CDCl
3):
δ 7.34–7.15 (m, 5H), 4.85 (dd,
J = 10.0, 5.4 Hz, 1H), 3.98 (dd,
J = 5.1, 2.4 Hz, 1H), 3.74 (s, 3H), 3.11, 3.15 (AMX,
J = 5.6, 5.1, 2.4 Hz, 2H), 3.24, 3.00 (AMX,
J = 14.4, 10.0, 5.4 Hz 2H), 1.86 (bs, 2H) ppm;
13C-NMR (100 MHz, CDCl
3):
δ 171.2, 170.5, 136.2, 128.8, 128.7, 127.3, 59.5, 54.8, 52.6, 49.4, 35.7 ppm; MS (ESI
+):
m/
z 271.2 [M + Na]
+; HRMS (ESI
+): Calc mass for C
13H
17N
2O
3: 249,1234; measured: 249.1232 [M + H]
+; IR (ATR): 3325.0, 2954.3, 1731.2, 1631.2, 1554.1, 1380.2, 1340.3, 1222.7, 1174.7, 744.3, 698.7 cm
−1;
= −41.8 (1.874, MeOH).
Methyl (R)-2-((S)-3-amino-2-oxoazetidin-1-yl)-3-(4-hydroxyphenyl)propanoate (
7d,
Scheme 3). Compound
7d was synthesized from
3d (4.30 g, 6.4 mmol) or
8 (2.00 g, 3.60 mmol) according to the procedure described for the preparation of
7c and purified with flash column chromatography on silica using CH
2Cl
2:MeOH = 9:1 as eluent. Tetrahedral white crystals (from
3d: 1.37 g, 5.2 mmol, 80% yield, from
8: 808 mg, 3.1 mmol, 85% yield); mp = 94–95 °C; R
f (CH
2Cl
2:MeOH = 9:1) = 0.21;
1H-NMR (400 MHz, CDCl
3):
δ 7.07 (d,
J = 8.5 Hz, 2H), 6.72 (d,
J = 8.5 Hz, 2H), 4.74 (dd,
J = 11.1, 4.9 Hz, 1H), 4.09 (dd,
J = 5.1, 2.3 Hz, 1H), 3.85 (bs, 2H), 3.76 (s, 3H), 3.72, 3.01 (AMX,
J = 5.8, 5.1, 2.3 Hz, 2H), 3.23, 2.89 (AMX,
J = 14.5, 11.1, 4.8 Hz 2H) ppm;
13C-NMR (100 MHz, CDCl
3):
δ 171.4, 170.5, 156.1, 130.1, 126.9, 115.9, 59.2, 54.7, 52.7, 49.2, 35.3 ppm; HRMS (ESI
+): Calc mass for C
13H
17N
2O
4 [M + H]
+: 265.1188; measured: 265.1184; IR (ATR): 3260.4, 1731.1, 1649.4, 1514.8, 1229.5, 1019.9, 825.1 cm
−1;
= +52.6 (0.61, MeOH).
Methyl (R)-2-(3-amino-2-oxoazetidin-1-yl)-2-(4-hydroxyphenyl)acetate (
7e,
Scheme 3). Compound
7e was synthesized from
1e (3.85 g, 7.4 mmol) according to the procedure described for the preparation of
7c and purified with flash column chromatography on silica using CH
2Cl
2:MeOH = 9:1 as eluent. Yellow gel (1.444 g, 5.77 mmol, 78% yield); R
f (DKM/MeOH= 9/1) = 0.29;
1H-NMR (400 MHz, MeOD):
δ 7.13–7.17 (m, 2H), 6.81–6.84 (m, 2H), 5.48 (s, 1H), 4.18 (dd,
J = 5.3, 2.5 Hz, 0.5H), 4.06 (dd,
J = 4.9, 2.5 Hz, 0.5H), 3.82 (t,
J = 5.3 Hz, 0.5H), 3.75 (s, 3H), 3.37–3.39 (m, 1H), 2.85–2.88 (m, 0.5H) ppm;
13C-NMR (100 MHz, MeOD):
δ 172.0; 171.7; 159.3; 130.8; 130.6; 125.2; 116.8; 59.6; 58.5; 53.1; 50.3 ppm; MS (ESI
+): 251.1 [M + H]
+; HRMS (ESI
+): Calc mass for C
12H
14O
4N
2: 251.1032, measured: 251.1038 [M + H]
+; IR (ATR): 2959.1, 1732.4, 1648.3, 1611.9, 1515.5, 1451.6, 1384.3, 1236.7, 1175.2, 1106.2, 1011.1, 833.4, 757.5, 528.9 cm
−1; HRMS (ESI
−): Calc mass for C
12H
13N
2O
4 [M − H]
−: 249.0881; measured: 249.0884.