Reduction of Preneoplastic Lesions Induced by 1,2-Dimethylhydrazine in Rat Colon by Maslinic Acid, a Pentacyclic Triterpene from Olea europaea L.
Abstract
:1. Introduction
2. Results
2.1. Body Weight, Food and Water Consumption, and Food Conversion Efficiency
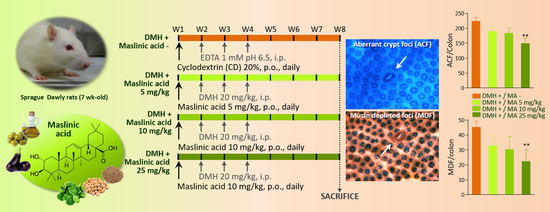
2.2. Aberrant Crypt Foci
2.3. Mucin-Depleted Foci
2.4. Determination of AST and ALT
2.5. Quantification of Maslinic Acid in Colon Content
2.6. Correlation between the Reductions of Preneoplastic Markers and Concentrations in the Colonic Content
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Diets
4.3. Experimental Design
4.4. Sample Collection
4.5. Aberrant Crypt Foci
4.6. Mucin Depleted Foci
4.7. Determination of AST and ALT
4.8. Determination of Maslinic Acid in Colon Content
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2018, 00, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Farinetti, A.; Zurlo, V.; Manenti, A.; Coppi, F.; Mattioli, A.V. Mediterranean diet and colorectal cancer: A systematic review. Nutrition 2017, 43, 83–88. [Google Scholar] [CrossRef]
- Haslam, A.; Robb, S.W.; Hébert, J.R.; Huang, H.; Ebell, M.H. Greater adherence to a Mediterranean diet is associated with lower prevalence of colorectal adenomas in men of all races. Nutr. Res. 2017, 48, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Boushey, C.J.; Wilkens, L.R.; Haiman, C.A.; Le Marchand, L. High-quality diets associate with reduced risk of colorectal cancer: Analyses of diet quality indexes in the multiethnic cohort. Gastroenterology 2017, 153, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Alfaras, I.; Juan, M.E.; Planas, J.M. trans-Resveratrol reduces precancerous colonic lesions in dimethylhydrazine-treated rats. J. Agric. Food Chem. 2010, 58, 8104–8110. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Khor, T.O.; Shen, G.; Jeong, W.S.; Hebbar, V.; Chen, C.; Xu, C.; Reddy, B.; Chada, K.; Kong, A.N. Cancer chemoprevention of intestinal polyposis in ApcMin/+ mice by sulforaphane, a natural product derived from cruciferous vegetable. Carcinogenesis 2006, 27, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Kumar, S.A.; Dharmalingam, P.; Ganapasam, S. Luteolin, a bioflavonoid inhibits azoxymethane-induced colon carcinogenesis: Involvement of iNOS and COX-2. Pharmacogn. Mag. 2014, 10, S306–S310. [Google Scholar] [PubMed]
- Neergheen, V.S.; Bahorun, T.; Taylor, E.W.; Jen, L.S.; Aruoma, O.I. Targeting specific cell signaling transduction pathways by dietary and medicinal phytochemicals in cancer chemoprevention. Toxicology 2010, 278, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Mena, G.; Sánchez-González, M.; Juan, M.E.; Planas, J.M. Maslinic acid, a natural phytoalexin-type triterpene from olives-a promising nutraceutical? Molecules 2014, 19, 11538–11559. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, M.; Lozano-Mena, G.; Juan, M.E.; García-Granados, A.; Planas, J.M. Assessment of the safety of maslinic acid, a bioactive compound from Olea europaea L. Mol. Nutr. Food Res. 2013, 57, 339–346. [Google Scholar] [CrossRef]
- Juan, M.E.; Wenzel, U.; Ruiz-Gutierrez, V.; Daniel, H.; Planas, J.M. Olive fruit extracts inhibit proliferation and induce apoptosis in HT-29 human colon cancer cells. J. Nutr. 2006, 136, 2553–2557. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Zurita, F.J.; Pachón-Peña, G.; Lizárraga, D.; Rufino-Palomares, E.E.; Cascante, M.; Lupiáñez, J.A. The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNK-p53-dependent mechanism. BMC Cancer 2011, 11, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; García-Salguero, L.; Peragón, J.; Medina, P.P.; Parra, A.; Cascante, M.; Lupiáñez, J.A. Maslinic acid, a natural triterpene, induces a death receptor-mediated apoptotic mechanism in Caco-2 p53-deficient colon adenocarcinoma cells. PloS ONE 2016, 11, e0146178–e0146194. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Mena, G.; Sánchez-González, M.; Parra, A.; Juan, M.E.; Planas, J.M. Identification of gut-derived metabolites of maslinic acid, a bioactive compound from Olea europaea L. Mol. Nutr. Food Res. 2016, 60, 2053–2064. [Google Scholar] [CrossRef]
- Sánchez-Tena, S.; Reyes-Zurita, F.J.; Díaz-Moralli, S.; Vinardell, M.P.; Reed, M.; García-Garía, F.; Dopazo, J.; Lupiáñez, J.A.; Günther, U.; Cascante, M. Maslinic acid-enriched diet decreases intestinal tumorigenesis in ApcMin/+ mice through transcriptomic and metabolomic reprogramming. PLoS ONE 2013, 8, e59392–e59403. [Google Scholar]
- Rosenberg, D.W.; Giardina, C.; Tanaka, T. Mouse models for the study of colon carcinogenesis. Carcinogenesis 2009, 30, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Femia, A.P.; Tarquini, E.; Salvadori, M.; Ferri, S.; Giannini, A.; Dolara, P.; Caderni, G. K-ras mutations and mucin profile in preneoplastic lesions and colon tumors induced in rats by 1,2-dimethylhydrazine. Int. J. Cancer 2008, 122, 117–123. [Google Scholar]
- Perše, M.; Cerar, A. Morphological and molecular alterations in 1,2 dimethylhydrazine and azoxymethane induced colon carcinogenesis in rats. J. Biomed. Biotechnol. 2011, 2011, 473964–473978. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Takahashi, M.; Wakabayashi, K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004, 95, 475–480. [Google Scholar] [CrossRef]
- Femia, A.P.; Dolara, P.; Giannini, A.; Salvadori, M.; Biggeri, A.; Caderni, G. Frequent mutation of Apc gene in rat colon tumors and mucin-depleted foci, preneoplastic lesions in experimental colon carcinogenesis. Cancer Res. 2007, 67, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.H.; Thulasingam, S.; Nagarajan, S. Terpenoids as anti-colon cancer agents—A comprehensive review on its mechanistic perspectives. Eur. J. Pharmacol. 2017, 795, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Femia, A.P.; Dolara, P.; Luceri, C.; Salvadori, M.; Caderni, G. Mucin-depleted foci show strong activation of inflammatory markers in 1,2-dimethylhydrazine-induced carcinogenesis and are promoted by the inflammatory agent sodium dextran sulfate. Int. J. Cancer 2009, 125, 541–547. [Google Scholar] [CrossRef]
- Raju, J. Azoxymethane-induced rat aberrant crypt foci: relevance in studying chemoprevention of colon cancer. World J. Gastroenterol. 2008, 21, 6632–6635. [Google Scholar] [CrossRef]
- Yan, S.L.; Yang, H.T.; Lee, H.L.; Yin, M.C. Protective effects of maslinic acid against alcohol-induced acute liver injury in mice. Food Chem. Toxicol. 2014, 74, 149–155. [Google Scholar] [CrossRef]
- Hidalgo, M.C.; Skalli, A.; Abellán, E.; Arizcun, M.; Cardenete, G. Dietary intake of probiotics and maslinic acid in juvenile dentex (Dentex dentex L.): Effects on growth performance, survival and liver proteolytic activities. Aquaculture Nutr. 2006, 12, 256–266. [Google Scholar] [CrossRef]
- Fukumitsu, S.; Villareal, M.O.; Aida, K.; Hino, A.; Hori, N.; Isoda, H.; Naito, Y. Maslinic acid in olive fruit alleviates mild knee joint pain and improves quality of life by promoting weight loss in the elderly. J. Clin. Biochem. Nutr. 2016, 59, 220–225. [Google Scholar] [CrossRef]
- Bird, R.P.; Good, C.K. The significance of aberrant crypt foci in understanding the pathogenesis of colon cancer. Toxicol. Lett. 2000, 112–113, 395–402. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Zuo, X.; Wang, M. Chemopreventive effects of some popular phytochemicals on human colon cancer: A review. Food Funct. 2018, 9, 4548–4568. [Google Scholar] [CrossRef] [PubMed]
- Kawamori, T.; Tanaka, T.; Hara, A.; Yamahara, J.; Mori, H. Modifying effects of naturally occurring products on the development of colonic aberrant crypt foci induced by azoxymethane in F344 rats. Cancer Res. 1995, 55, 1277–1282. [Google Scholar] [PubMed]
- Janakiram, N.B.; Indranie, C.; Malisetty, S.V.; Jagan, P.; Steele, V.E.; Rao, C.V. Chemoprevention of colon carcinogenesis by oleanolic acid and its analog in male F344 rats and modulation of COX-2 and apoptosis in human colon HT-29 cancer cells. Pharm. Res. 2008, 25, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Furtado, R.A.; Rodrigues, E.P.; Araújo, F.R.; Oliveira, W.L.; Furtado, M.A.; Castro, M.B.; Cunha, W.R.; Tavares, D.C. Ursolic acid and oleanolic acid suppress preneoplastic lesions induced by 1,2-dimethylhydrazine in rat colon. Toxicol Pathol. 2008, 36, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Khan, A.Q.; Lateef, A.; Rehman, M.U.; Tahir, M.; Ali, F.; Hamiza, O.O.; Sultana, S. Glycyrrhizic acid suppresses the development of precancerous lesions via regulating the hyperproliferation, inflammation, angiogenesis and apoptosis in the colon of Wistar rats. PloS ONE 2013, 8, e56020–e56042. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, Z.; Zhai, C.; Qiu, W.; Li, D.; Yi, Z.; Wang, L.; Tang, J.; Qian, M.; Luo, J.; Liu, M. Maslinic acid potentiates the anti-tumor activity of tumor necrosis factor alpha by inhibiting NF-kappaB signaling pathway. Mol. Cancer 2010, 9, 73–86. [Google Scholar] [CrossRef]
- Hsum, Y.W.; Yew, W.T.; Hong, P.L.; Soo, K.K.; Hoon, L.S.; Chieng, Y.C.; Mooi, L.Y. Cancer chemopreventive activity of maslinic acid: Suppression of COX-2 expression and inhibition of NF-κB and AP-1 activation in Raji cells. Planta Med. 2011, 77, 152–157. [Google Scholar] [CrossRef]
- Juan, M.E.; Planas, J.M.; Ruiz-Gutiérrez, V.; Daniel, H.; Wenzel, U. Antiproliferative and apoptosis-inducing effects of maslinic and oleanolic acids, two pentacyclic triterpenes from olives, on HT-29 colon cancer cells. Br. J. Nutr. 2008, 100, 36–43. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; Medina, P.P.; García-Salguero, E.L.; Peragón, J.; Cascante, M.; Lupiáñez, J.A. Antitumour activity on extrinsic apoptotic targets of the triterpenoid maslinic acid in p53-deficient Caco-2 adenocarcinoma cells. Biochimie 2013, 95, 2157–2167. [Google Scholar] [CrossRef]
- Ratjen, I.; Schafmayer, C.; di Giuseppe, R.; Waniek, S.; Plachta-Danielzik, S.; Koch, M.; Nöthlings, U.; Hampe, J.; Schlesinger, S.; Lieb, W. Postdiagnostic mediterranean and healthy nordic dietary patterns are inversely associated with all-cause mortality in long-term colorectal cancer survivors. J. Nutr. 2017, 147, 636–644. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Ioannou, M.; Karathanos, V.T.; Hassapidou, M.; Andrikopoulos, N.K. Nutritional evaluation and bioactive microconstituents (phytosterols, tocopherols, polyphenols, triterpenic acids) in cooked dry legumes usually consumed in the Mediterranean countries. Food Chem. 2010, 121, 682–690. [Google Scholar] [CrossRef]
- Lin, C.C.; Huang, C.Y.; Mong, M.C.; Chan, C.Y.; Yin, M.C. Antiangiogenic potential of three triterpenic acids in human liver cancer cells. J. Agric. Food Chem. 2011, 59, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; García, A.; Medina, E.; Ruíz-Méndez, M.A.; de Castro, A.; Brenes, M. Triterpenic acids in table olives. Food Chem. 2010, 118, 670–674. [Google Scholar] [CrossRef]
- Bird, R.P. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987, 37, 147–151. [Google Scholar] [CrossRef]
- Caderni, G.; Femia, A.P.; Giannini, A.; Favuzza, A.; Luceri, C.; Salvadori, M.; Dolara, P. Identification of mucin-depleted foci in the unsectioned colon of azoxymethane-treated rats: correlation with carcinogenesis. Cancer Res. 2003, 63, 2388–2392. [Google Scholar] [PubMed]








| DMH−/MA− | DMH+/MA− | DMH+/MA 25 | |
|---|---|---|---|
| AST, UI/L | 161 ± 20 (n = 8) | 172 ± 30 (n = 8) | 178 ± 15 (n = 6) |
| ALT, UI/L | 54.8 ± 2.7 (n = 8) | 53.5 ± 3.9 (n = 8) | 54.1 ± 2.8 (n = 6) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juan, M.E.; Lozano-Mena, G.; Sánchez-González, M.; Planas, J.M. Reduction of Preneoplastic Lesions Induced by 1,2-Dimethylhydrazine in Rat Colon by Maslinic Acid, a Pentacyclic Triterpene from Olea europaea L. Molecules 2019, 24, 1266. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24071266
Juan ME, Lozano-Mena G, Sánchez-González M, Planas JM. Reduction of Preneoplastic Lesions Induced by 1,2-Dimethylhydrazine in Rat Colon by Maslinic Acid, a Pentacyclic Triterpene from Olea europaea L. Molecules. 2019; 24(7):1266. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24071266
Chicago/Turabian StyleJuan, M. Emília, Glòria Lozano-Mena, Marta Sánchez-González, and Joana M. Planas. 2019. "Reduction of Preneoplastic Lesions Induced by 1,2-Dimethylhydrazine in Rat Colon by Maslinic Acid, a Pentacyclic Triterpene from Olea europaea L." Molecules 24, no. 7: 1266. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24071266