Multi-Modal Compositional Analysis of Layered Paint Chips of Automobiles by the Combined Application of ATR-FTIR Imaging, Raman Microspectrometry, and SEM/EDX
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Sample Preparation for ATR-FTIR Imaging and RMS Measurements
2.3. ATR-FTIR Imaging Measurements
2.4. RMS Measurements
2.5. SEM/EDX Measurements
3. Results and Discussion
3.1. Characterization of Sample A
3.1.1. Characterization of Layers #1, #2, #4, and #5 (Alkyd and Alkyd-Melamine Resins)
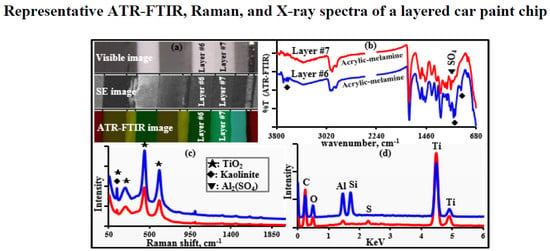
3.1.2. Characterization of Layers #3, #6, and #7 (Acrylic-Melamine Resins)
3.1.3. Characterization of Layers #8 and #9 (Epoxy Resins)
3.2. Characterization of Sample B
3.2.1. Characterization of Layer #1 (Ester-Epoxy Resin)
3.2.2. Characterization of Layers #2, #3, and #4 (Alkyd-Melamine Resins)
3.2.3. Characterization of Layer #5 (Butyl Acrylic Resin)
3.3. Characterization of Sample C
3.3.1. Characterization of Layers #1 and #2 (Polybutadiene Resin)
3.3.2. Characterization of Layers #3 and #4 (Acrylic Resin)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Scientific Working Group on Materials Analysis (SWGMAT). Forensic paint analysis and comparison guidelines. Forensic Sci. Commun. 1999, 1, 1–28. [Google Scholar]
- Jariwala, K.N. Forensic examination of paint. Indian J. Appl. Res. 2014, 4, 247–248. [Google Scholar] [CrossRef]
- Deconinck, I.; Latkoczy, C.; Gunther, D.; Govaerf, F.; Vanhaecke, F. Capabilities of laser ablation-inductively coupled plasma mass spectrometry for (trace) element analysis of car paints for forensic purposes. J. Anal. At. Spectrom. 2006, 21, 279–287. [Google Scholar]
- McIntee, E.; Viglino, E.; Rinke, C.; Kumor, S.; Ni, L.; Sigman, M.E. Comparative analysis of automotive paints by laser induced breakdown spectroscopy and nonparametric permutation tests. Spectrochim. Acta B 2010, 65, 542–548. [Google Scholar] [CrossRef]
- Zieba-Palus, J.; Zadora, G.; Milczarek, J.M.; Koscielniak, P. Pyrolysis-gas chromatography/mass spectrometry analysis as a useful tool in forensic examination of automotive paint traces. J. Chromatogr. A 2008, 1179, 41–46. [Google Scholar] [CrossRef]
- Lee, Y.; Han, S.; Yoon, J.-H.; Kim, Y.-M.; Shon, S.-K.; Park, S.-W. Application of time-of-flight secondary ion mass spectrometry to automobile paint analysis. Anal. Sci. 2001, 17, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Nishi, C.; Otsuki, K.; Nishiwaki, Y.; Tsuji, K. Depth elemental imaging of forensic samples by confocal micro-XRF method. Anal. Chem. 2011, 83, 3477–3483. [Google Scholar] [CrossRef]
- Sun, T.; Liu, Z.; Wang, G.; Ma, Y.; Peng, S.; Sun, W.; Li, F.; Sun, X.; Ding, X. Application of confocal X-ray fluorescence micro-spectroscopy to the investigation of paint layers. Appl. Radiat. Isotopes 2014, 94, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Zadora, G.; Brozek-Mucha, Z. SEM-EDX a useful tool for forensic examinations. Mater. Chem. Phys. 2003, 81, 345–348. [Google Scholar] [CrossRef]
- Lambert, D.; Muehlethaler, C.; Gueissaz, L.; Massonnet, G. Raman analysis of multilayer automotive paints in forensic science: Measurement variability and depth profile. J. Raman Spectrosc. 2014, 45, 1285–1292. [Google Scholar] [CrossRef]
- Flynn, K.; O’Leary, R.; Lennard, C.; Roux, C.; Reedy, B.J. Forensic application of infrared chemical imaging: Multi-layered paint chips. J. Forensic Sci. 2005, 50, 1–10. [Google Scholar] [CrossRef]
- Maric, M.; Van Bronswijk, W.; Lewis, S.W.; Pitts, K. Synchrotron FTIR characterization of automotive primer surface paint coatings for forensic purposes. Talanta 2014, 118, 156–161. [Google Scholar] [CrossRef]
- Maric, M.; Van Bronswijk, W.; Lewis, S.W.; Pitts, K. Rapid characterization and classification of automotive clear coats by attenuated total reflectance infrared spectroscopy. Anal. Methods 2012, 4, 2687–2693. [Google Scholar] [CrossRef]
- Allauddin, S.; Chandran, M.K.A.; Jena, K.K.; Narayan, R.; Raju, K.V.S.N. Synthesis and characterization of APTMS/melamine cured hyperbranched polyester-epoxy hybrid coatings. Prog. Org. Coat. 2013, 76, 1402–1412. [Google Scholar] [CrossRef]
- Zieba-palus, J. Application of micro-Fourier transform infrared spectroscopy to the examination of paint samples. J. Mol. Struct. 1999, 511–512, 327–335. [Google Scholar] [CrossRef]
- Suzuki, E.M. Infrared spectra of U.S. automobile original finishes (1998-2000). IX. Identification of Bismuth oxychloride and silver/white mica pearlescent pigments using extended range FT-IR spectroscopy, XRF spectroscopy, and SEM/EDS analysis. J. Forensic Sci. 2014, 59, 1205–1225. [Google Scholar] [CrossRef] [PubMed]
- Otieno-Alego, V. Some forensic applications of a combined micro-Raman and scanning electron microscopy system. J. Raman Spectrosc. 2009, 40, 948–953. [Google Scholar] [CrossRef]
- Zieba-Palus, J.; Borusiewicz, R. Examination of multilayer paint coats by the use of infrared, Raman, and XRF spectroscopy for forensic purposes. J. Mol. Struct. 2006, 792–793, 286–292. [Google Scholar] [CrossRef]
- Zeiba-Palus, J.; Trzcinska, B.M. Application of infrared and Raman spectroscopy in paint trace examination. J. Forensic Sci. 2013, 58, 1359–1363. [Google Scholar] [CrossRef]
- Renishaw plc. Raman and FTIR Analysis of a Forensic Paint Chip Sample: Application Note #SPD/AN/104; Renishaw plc.: Gloucestershire, UK, 2006. [Google Scholar]
- He, J.; Lv, J.; Ji, Y.; Feng, J.; Liu, Y. Multiple characterization of automotive coatings in forensic analysis. Spectrosc. Lett. 2013, 46, 555–560. [Google Scholar] [CrossRef]
- Robertson, I. Analysis of Automobile Paint Chips Using an Automated IR Microscope: Application Note #012077_01; PerkinElmer, Inc.: Waltham, MA, USA, 2015. [Google Scholar]
- Bruker Optics, Inc. Analysis of Multi-Layered Paint Chips: Application Note #BOPT 4000365-01; Bruker Optics, Inc.: Billerica, MA, USA, 2011. [Google Scholar]
- PerkinElmer, Inc. Large Area ATR-FTIR Imaging Using the Spotlight FTIR Imaging System: Technical Note #007641_02; PerkinElmer, Inc.: Shelton, CT, USA, 2006. [Google Scholar]
- Joseph, E.; Ricci, C.; Kazarian, S.G.; Mazzeo, R.; Prati, S.; Ioele, M. Macro-ATR-FTIR spectroscopic imaging analysis of paint cross-sections. Vib. Spectrosc. 2010, 53, 274–278. [Google Scholar] [CrossRef]
- Ryu, J.-Y.; Ro, C.-U. Attenuated total reflectance FTIR imaging and quantitative energy dispersive-electron probe X-ray microanalysis techniques for single particle analysis of atmospheric aerosol particles. Anal. Chem. 2009, 81, 6695–6707. [Google Scholar] [CrossRef]
- Malek, M.A.; Eom, H.-J.; Hwang, H.; Hur, S.D.; Hong, S.; Hou, S.; Ro, C.-U. Single particle mineralogy of microparticles from Himalayan ice-cores using SEM/EDX and ATR-FTIR imaging techniques for identification of volcanic ash signatures. Chem. Geol. 2019, 504, 205–215. [Google Scholar] [CrossRef]
- PerkinElmer, Inc. Spatial Resolution in ATR-FTIR Imaging: Measurement and Interpretation: Technical Note #007641_03; PerkinElmer, Inc.: Shelton, CN, USA, 2006. [Google Scholar]
- Sobanska, S.; Hwang, H.; Choël, M.; Jung, H.; Eom, H.-J.; Kim, H.; Barbillat, J.; Ro, C.-U. Investigation of the chemical mixing state of individual Asian Dust particles by the combined use of Electron Probe X-ray Microanalysis and Raman Microspectrometry. Anal. Chem. 2012, 84, 3145–3154. [Google Scholar] [CrossRef]
- Jung, H.J.; Eom, H.-J.; Kang, H.W.; Moreau, M.; Sobanska, S.; Ro, C.-U. Combined use of quantitative ED-EPMA, Raman microspectrometry, and ATR-FTIR imaging techniques for the analysis of individual particles. Analyst 2014, 139, 3949–3960. [Google Scholar] [CrossRef] [PubMed]
- Eom, H.-J.; Gupta, D.; Cho, H.-R.; Hwang, H.; Hur, S.D.; Hong, S.; Kim, Y.; Ro, C.-U. Single-particle investigation of summertime and wintertime Antarctic sea spray aerosols using low-Z particle EPMA, Raman microspectrometry, and ATR-FTIR imaging techniques. Atmos. Chem. Phys. 2016, 16, 13823–13836. [Google Scholar] [CrossRef] [Green Version]
- Ro, C.-U.; Osan, J.; Van Grieken, R. Determination of low-Z elements in individual environmental particles using windowless EPMA. Anal. Chem. 1999, 71, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.A.; Abu-Sbeih, K.A.; Al-Trawneh, I.; Bourghli, L. Preparation and characterization of alkyd resins of Jordan valley tomato oil. J. Polym. Environ. 2014, 22, 553–558. [Google Scholar] [CrossRef]
- Cakic, S.M.; Boskovis, L.B. FTIR analysis and the effects of alkyd/melamine resin ratio on the properties of the coatings. Hem. Ind. 2009, 63, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Gan, S.-N.; Tan, B.-Y. FTIR studies of the curing reactions of palm oil alkyd-melamine enamels. J. Appl. Polym. Sci. 2001, 80, 2309–2315. [Google Scholar] [CrossRef]
- Bell, S.E.J.; Fido, L.A.; Speers, S.J.; Armstrong, W.J.; Spratt, S. Forensic analysis of architectural finishes using Fourier transform infrared and Raman spectroscopy. Appl. Specrosc. 2005, 59, 1333–1339. [Google Scholar] [CrossRef]
- Scheepers, M.L.; Meier, R.J.; Markwort, L.; Gelan, J.M.; Vanderzande, D.J.; Kip, B.J. Determination of free melamine content in melamine-formaldehyde resins by Raman spectroscopy. Vib. Spectrosc. 1995, 9, 139–146. [Google Scholar] [CrossRef]
- Mircescu, N.E.; Oltean, M.; Chis, V.; Leopold, N. FTIR, FT-Raman, SERS and DFT study on melamine. Vib. Spectrosc. 2012, 62, 165–171. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Zhang, S.; Gao, Q.; Li, J. Effects of melamine addition stage on the performance and curing behavior of melamine-urea-formaldehyde (MUF) resin. BioResources 2013, 8, 5500–5514. [Google Scholar] [CrossRef]
- Ricciotti, L.; Roviello, G.; Tarallo, O.; Borbone, F.; Ferone, C.; Colangelo, F.; Catauro, M.; Cioffi, R. Synthesis and characterization of melamine-based epoxy resin. Int. J. Mol. Sci. 2013, 14, 18200–18214. [Google Scholar] [CrossRef] [PubMed]
- Nishino, G.; Sugimoto, H.; Nakanishi, E.J. Preparation and properties of acrylic melamine hard coating. Appl. Polym. Sci. 2012, 123, 307–315. [Google Scholar] [CrossRef]
- Hirayama, T.; Urban, M.W. Distribution of melamine in melamine/polyester coatings; FTIR spectroscopic studies. Prog. Org. Coat. 1992, 20, 81–96. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Smith, B. Hydrocarbon. In Infrared Spectral Interpretation: A Systematic Approach; Smith, B., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 31–66. [Google Scholar]
- Bartolozzi, G.; Marchiafava, V.; Mirabello, V.; Peruzzini, M.; Picollo, M. Chemical curing in alkyd paints: An evaluation via FTIR and NMR spectroscopies. Spectrochim. Acta A 2014, 118, 520–525. [Google Scholar] [CrossRef]
- Ploeger, R.; Chiantore, O. Characterization and Stability Issues of Artists’ Alkyd Paints; Smithsonian Contribution Series: Smithsonian Contribution to Museum Conservation, Number 3: New Insights into the Cleaning of Paintings; Smithsonian Institution: Washington, DC, USA, 2013; pp. 89–95. [Google Scholar]
- Smith, B. Carbonyl functional group. In Infrared Spectral Interpretation: A Systematic Approach; Smith, B., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 91–115. [Google Scholar]
- Wijnhoven, E.G.J.; Vos, W.L. Preparation of photonic crystal made of air sphere in Titania. Science 1998, 281, 802–804. [Google Scholar] [CrossRef]
- Learner, T.; Gallery, T. The use of a diamond cell for the FTIR characterization of paints and varnishes available to twentieth century artist. In Proceedings of the IRUG 2 Conference, Victoria and Albert Museum (V&A), London, UK, 12–13 September 1995; pp. 7–20. [Google Scholar]
- Emmons, E.D.; Kraus, R.G.; Duvvuri, S.S.; Thompson, J.S.; Covington, A.M. High-pressure infrared absorption spectroscopy of poly(methyl methacrylate). J. Polym. Sci. B 2007, 45, 358–367. [Google Scholar] [CrossRef]
- Muril, C.; Song, Y. Pressure-induced polymerization of acrylic acid: A Raman spectroscopic study. J. Phys. Chem. B 2010, 114, 9744–9750. [Google Scholar] [CrossRef]
- Jung, H.-J.; Malek, M.A.; Ryu, J.-Y.; Kim, B.; Song, Y.-C.; Kim, H.K.; Ro, C.-U. Speciation of individual mineral particles of micrometer size by the combined use of ATR-FTIR imaging and quantitative ED-EPMA techniques. Anal. Chem. 2010, 82, 6193–6202. [Google Scholar] [CrossRef]
- Balan, E.; Saitta, A.M.; Mauri, F.; Calas, G. First-principles modeling of the infrared spectrum of kaolinite. Am. Mineral. 2001, 86, 1321–1330. [Google Scholar] [CrossRef]
- Eom, H.-J.; Jung, H.-J.; Sobanska, S.; Chung, S.-G.; Son, Y.-S.; Kim, J.-C.; Sunwoo, Y.; Ro, C.-U. Iron speciation of airborne subway particles by the combined use of ED-EPMA and Raman microspectrometry. Anal. Chem. 2013, 85, 10424–10431. [Google Scholar] [CrossRef]
- González, M.G.; Cabanelas, J.C.; Baselga, J. Applications of FTIR on epoxy resins- identification, monitoring, the curing process, phase separation and water uptake. In Infrared Spectroscopy: Materials Science, Engineering and Technology; Theophanides, T., Ed.; InTech: Rijeka, Croatia, 2012; pp. 261–284. [Google Scholar]
- Nikolic, G.; Zlatkovic, S.; Cakic, M.; Cakic, S.; Lacnjevac, C.; Rajic, Z. Fast FTIR characterization of epoxy GY system crosslinked with aliphatic and cycloaliphatic EH polyamine adducts. Sensors 2010, 10, 684–696. [Google Scholar] [CrossRef]
- Remazeilles, C.; Saheb, M.; Neff, D.; Guilminot, E.; Tran, K.; Bourdoiseau, J.-A.; Sabot, R.; Jeannin, M.; Matthiesen, H.; Dillmann, P.; et al. Microbiologically influenced corrosion of archaeological artefacts: Characterization of iron(II) sulfides by Raman spectroscopy. J. Raman Spectrosc. 2010, 41, 1425–1433. [Google Scholar] [CrossRef]
- Dieguez, A.; Romano-Rodriguez, A.; Vila, A.; Morante, J.R. The complete Raman spectrum of nanometric SnO2 particles. J. Appl. Phys. 2001, 90, 1550–1557. [Google Scholar] [CrossRef]
- Stepakova, L.V.; Skripkin, M.Y.; Chernykh, L.V.; Starova, G.L.; Hajba, L.; Mink, J.; Sandstrom, M. Vibrational spectroscopic and force field studies of copper(II) chloride and bromide compounds, and crystal structure of KCuBr3. J. Raman Spectrosc. 2008, 39, 16–31. [Google Scholar] [CrossRef]
- Shin, H.S.; Jung, Y.M.; Lee, J.; Chang, T.; Ozaki, Y.; Kim, S.B. Structural comparison of Langmuir-Blodgett and spin-coated films of poly(tert-butyl methacrylate) by external reflection FTIR spectroscopy and two-dimensional correlation analysis. Langmuir 2002, 18, 5523–5528. [Google Scholar] [CrossRef]
- Jingshui, X.; Yangchuan, K.; Qian, Z.; Xianglong, H. Synthesis and properties of poly (butyl acrylate-co-methyl methacrylate) copolymer microspheres of tunable size using suspension polymerization. Compos. Interface 2013, 20, 165–176. [Google Scholar] [CrossRef]
- Arenas, J.F.; Castro, J.L.; Otero, J.C.; Marcos, J.I. SERS of acrylic acid and methyl derivatives on silver sols. J. Raman Spectrosc. 1998, 29, 585–591. [Google Scholar] [CrossRef]
- Munteanu, S.B.; Vasile, C.J. Spectral and thermal characterization of styrene-butadiene copolymers with different architectures. Optoelectron. Adv. Mater. 2005, 7, 3135–3148. [Google Scholar]
- Cai, M.; Pan, X.; Liu, W.; Sheng, Z.; Fang, X.; Zhang, C.; Huo, Z.; Tian, H.; Xiao, S.; Dai, S. Multiple adsorption of tributyl phosphate molecule at the dyed-TiO2/electrolyte interface to suppress the charge recombination in dye-sensitized solar cell. J. Mater. Chem. A 2013, 1, 4885–4892. [Google Scholar] [CrossRef]
- Nallasamy, P.; Anbarasan, P.M.; Mohan, S. Vibrational spectra and assignments of cis- and trans-1,4-polybutadiene. Turk. J. Chem. 2002, 26, 105–111. [Google Scholar]
Sample Availability: The car paint chip samples are available from the authors. |









| Sample | Layer # | Elements from EDX | ATR-FTIR and RMS Assignment | |
|---|---|---|---|---|
| Major | Minor | |||
| A | #1 | C, O | Enamel or alkyd resin | Polyalkoxylated phenol |
| #2 | C, N, O, Ti, Al-flakes | Alkyd-melamine resin | Rutile (TiO2), Al-flakes | |
| #3 | C, N, O, Ti, Al, Si, Na | Acrylic-melamine resin | Rutile, Kaolinite | |
| #4 | C, O | Enamel or alkyd resin | Polyalkoxylated phenol | |
| #5 | C, N, O, Ti, Al | Alkyd-melamine resin | Rutile, Al-flakes | |
| #6 | C, N, O, Ti, Al, Si, Na | Acrylic-melamine resin | Rutile, Kaolinite | |
| #7 | C, N, O, Ti, Al, Mg, Si, S | Acrylic-melamine resin | Rutile, talc, Al2(SO4)3 | |
| #8 | C, N, O, Ti, Al, Si, Zn, Sn | Ester-epoxy resin | Pyrophyllite, SnO2 | |
| #9 | C, N, O, Al, Si, Ti, Fe, Zn, P | Non-ester epoxy resin | Kaolinite, Zn3(PO4)2 | |
| ZnO, Fe3O4 | ||||
| B | #1 | C, N, O, Al, Si, Ti, S, Sn | Ester-epoxy resin | Kaolinite, rutile, SnO2 |
| #2 | C, N, O, Ba, S, Al, Si, Fe | Alkyd-melamine resin-I | BaSO4 | |
| #3 | C, N, O, Ba, S, Cu, Cl | Alkyd-melamine resin-I | BaSO4, CuCl2 | |
| #4 | C, N, O | Alkyd-melamine resin-II | -- | |
| #5 | C, N, O, Al | Acrylic (butyl) resin | Al2O3 | |
| C | #1 | C, O, Mg, Si, Ca, Ti, Al | Polybutadiene + TBP | Talc, CaCO3, rutile |
| #2 | C, O, Mg, Si, Ca, Ti, Al | Polybutadiene + TBP | Talc, CaCO3, rutile | |
| #3 | C, O, Al, Ti, Fe | Acrylic copolymer | Al-flakes | |
| #4 | C, N, O | Acrylic copolymer | -- | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malek, M.A.; Nakazawa, T.; Kang, H.-W.; Tsuji, K.; Ro, C.-U. Multi-Modal Compositional Analysis of Layered Paint Chips of Automobiles by the Combined Application of ATR-FTIR Imaging, Raman Microspectrometry, and SEM/EDX. Molecules 2019, 24, 1381. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24071381
Malek MA, Nakazawa T, Kang H-W, Tsuji K, Ro C-U. Multi-Modal Compositional Analysis of Layered Paint Chips of Automobiles by the Combined Application of ATR-FTIR Imaging, Raman Microspectrometry, and SEM/EDX. Molecules. 2019; 24(7):1381. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24071381
Chicago/Turabian StyleMalek, Md Abdul, Takashi Nakazawa, Hyun-Woo Kang, Kouichi Tsuji, and Chul-Un Ro. 2019. "Multi-Modal Compositional Analysis of Layered Paint Chips of Automobiles by the Combined Application of ATR-FTIR Imaging, Raman Microspectrometry, and SEM/EDX" Molecules 24, no. 7: 1381. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24071381