The Influence of Various N-Heterocyclic Carbene Ligands on Activity of Nitro-Activated Olefin Metathesis Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of the Ruthenium Complexes
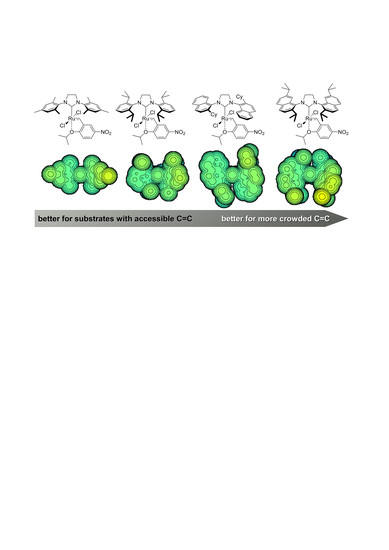
2.2. Structure Analysis
2.3. Comparative Catalytic Activity Studies of Nitro-Catalysts 4a–e
3. Experimental Section
3.1. General
3.2. Synthesis of Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wanzlick, H.-W.; Schönherr, H.-J. Direct Synthesis of a Mercury Salt-Carbene Complex. Angew. Chem. Int. Ed. 1968, 7, 141–142. [Google Scholar] [CrossRef]
- Öfele, K. 1,3-Dimethyl-4-imidazolinyliden-(2)-pentacarbonylchrom ein neuer übergangsmetall-carben-komplex. J. Organomet. Chem. 1968, 12, P42–P43. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Harlow, R.L.; Kline, M. A stable crystalline carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Gómez-Suárez, A.; Nelson, D.J.; Nolan, S.P. Quantifying and understanding the steric properties of N-heterocyclic carbenes. Chem. Commun. 2017, 53, 2650–2660. [Google Scholar] [CrossRef] [Green Version]
- Cazin, C. (Ed.) N-Heterocyclic Carbenes in Transition Metal Catalysis and Organocatalysis; Springer: Dordrecht, The Netherlands, 2011; Volume 32, pp. 1–336. [Google Scholar]
- Grubbs, R.H.; Wenzel, A.G.; O’Leary, D.J.; Khosravi, E. Handbook of Metathesis; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Grela, K. Olefin Metathesis: Theory and Practice; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Michrowska, A.; Grela, K. Quest for the ideal olefin metathesis catalyst. Pure Appl. Chem. 2008, 80, 31–43. [Google Scholar] [CrossRef]
- Anderson, A.W.; Merckling, N.G. Polymeric Bicyclo-(2, 2, 1)-2-Heptene. U.S. Patent 2,721,189, 18 October 1955. [Google Scholar]
- Banks, R.L.; Bailey, G.C. Olefin Disproportionation. A New Catalytic Process. Ind. Eng. Chem. Prod. Res. Dev. 1964, 3, 170–173. [Google Scholar] [CrossRef]
- Natta, G.; Dall’Asta, G.; Mazzanti, G. Stereospecific Homopolymerization of Cyclopentene. Angew. Chem. Int. Ed. 1964, 3, 723–729. [Google Scholar] [CrossRef]
- Schrock, R.R.; Murdzek, J.S.; Bazan, G.C.; Robbins, J.; Dimare, M.; O’Regan, M. Synthesis of molybdenum imido alkylidene complexes and some reactions involving acyclic olefins. J. Am. Chem. Soc. 1990, 112, 3875–3886. [Google Scholar] [CrossRef]
- Schwab, P.; Grubbs, R.H.; Ziller, J.W. Synthesis and Applications of RuCl2(CHR‘)(PR3)2: The Influence of the Alkylidene Moiety on Metathesis Activity. J. Am. Chem. Soc. 1996, 118, 100–110. [Google Scholar] [CrossRef]
- Ackermann, L.; Fürstner, A.; Weskamp, T.; Kohl, F.J.; Herrmann, W.A. Ruthenium carbene complexes with imidazolin-2-ylidene ligands allow the formation of tetrasubstituted cycloalkenes by RCM. Tetrahedron Lett. 1999, 40, 4787–4790. [Google Scholar] [CrossRef]
- Huang, J.; Stevens, E.D.; Nolan, S.P.; Petersen, J.L. Olefin Metathesis-Active Ruthenium Complexes Bearing a Nucleophilic Carbene Ligand. J. Am. Chem. Soc. 1999, 121, 2674–2678. [Google Scholar] [CrossRef]
- Scholl, M.; Trnka, T.M.; Morgan, J.P.; Grubbs, R.H. Increased ring closing metathesis activity of ruthenium-based olefin metathesis catalysts coordinated with imidazolin-2-ylidene ligands. Tetrahedron Lett. 1999, 40, 2247–2250. [Google Scholar] [CrossRef]
- Available online: https://pmc.umicore.com/en/products/umicore-grubbs-catalyst-m2a-c848/ (accessed on 15 April 2020).
- Scholl, M.; Ding, S.; Lee, C.W.; Grubbs, R.H. Synthesis and activity of a new generation of ruthenium-based olefin metathesis catalysts coordinated with 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene ligands. Org. Lett. 1999, 1, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://catalysts.evonik.com/product/catalysts/downloads/homogeneous_catalysts_evonik.pdf (accessed on 15 April 2020).
- Available online: https://pmc.umicore.com/en/products/umicore-grubbs-catalyst-m2/ (accessed on 15 April 2020).
- Available online: https://www.sigmaaldrich.com/catalog/product/sial/901755?lang=pl®ion=PL (accessed on 15 April 2020).
- Available online: https://www.strem.com/catalog/v/44-0758/59/ruthenium_502964-52-5 (accessed on 15 April 2020).
- Available online: https://www.strem.com/catalog/v/44-0770/59/ruthenium_928795-51-1 (accessed on 15 April 2020).
- Yang, L.; Mayr, M.; Wurst, K.; Buchmeiser, M.R. Novel metathesis catalysts based on ruthenium 1,3-dimesityl-3,4,5,6-tetrahydropyrimidin-2-ylidenes: Synthesis, structure, immobilization, and catalytic activity. Chem. Eur. J. 2004, 10, 5761–5770. [Google Scholar] [CrossRef]
- Despagnet-Ayoub, E.; Grubbs, R.H. A ruthenium olefin metathesis catalyst with a four-membered N-heterocyclic carbene ligand. Organometallics 2005, 24, 338. [Google Scholar] [CrossRef] [Green Version]
- Fürstner, A.; Ackermann, L.; Gabor, B.; Goddard, R.; Lehmann, C.W.; Mynott, R.; Stelzer, F.; Thiel, O.R. Comparative investigation of ruthenium-based metathesis catalysts bearing N-heterocyclic carbene (NHC) ligands. Chem.-A Eur. J. 2001, 7, 3236–3253. [Google Scholar] [CrossRef]
- Dinger, M.B.; Nieczypor, P.; Mol, J.C. Adamantyl-Substituted N-Heterocyclic Carbene Ligands in Second-Generation Grubbs-Type Metathesis Catalysts. Organometallics 2003, 22, 5291. [Google Scholar] [CrossRef]
- Yun, J.; Marinez, E.R.; Grubbs, R.H. A new ruthenium-based olefin metathesis catalyst coordinated with 1, 3-dimesityl-1, 4, 5, 6-tetrahydropyrimidin-2-ylidene: synthesis, X-ray structure, and reactivity. Organometallics 2004, 23, 4172. [Google Scholar] [CrossRef] [Green Version]
- Vehlow, K.; Maechling, S.; Blechert, S. Ruthenium metathesis catalysts with saturated unsymmetrical N-heterocyclic carbene ligands. Organometallics 2006, 25, 25. [Google Scholar] [CrossRef]
- Ledoux, N.; Allaert, B.; Pattyn, S.; Vander Mierde, H.; Vercaemst, C.; Verpoort, F. N,N′-Dialkyl- and N-Alkyl-N-mesityl-Substituted N-Heterocyclic Carbenes as Ligands in Grubbs Catalysts. Chem. Eur. J. 2006, 12, 4654. [Google Scholar] [CrossRef]
- Tornatzky, J.; Kannenberg, A.; Blechert, S. New catalysts with unsymmetrical N-heterocyclic carbene ligands. Dalton Trans. 2012, 41, 8215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamad, F.B.; Sun, T.; Xiao, S.; Verpoort, F. Olefin metathesis ruthenium catalysts bearing unsymmetrical heterocylic carbenes. Coord. Chem. Rev. 2013, 257, 2274–2292. [Google Scholar] [CrossRef]
- Paradiso, V.; Costabile, C.; Grisi, F. Ruthenium-based olefin metathesis catalysts with monodentate unsymmetrical NHC ligands. Beilstein J. Org. Chem. 2018, 14, 3122–3149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinger, M.B.; Mol, J.C. High turnover numbers with ruthenium-based metathesis catalysts. Adv. Synth. Catal. 2002, 344, 671–677. [Google Scholar] [CrossRef]
- Banti, D.; Mol, J.C. Degradation of the ruthenium-based metathesis catalyst [RuCl2 (CHPh)(H2IPr)(PCy3)] with primary alcohols. J. Organomet. Chem. 2004, 689, 3113. [Google Scholar] [CrossRef]
- Clavier, H.; Urbina-Blanco, C.A.; Nolan, S.P. Indenylidene Ruthenium Complex Bearing a Sterically Demanding NHC Ligand: An Efficient Catalyst for Olefin Metathesis at Room Temperature. Organometallics 2009, 28, 2848–2854. [Google Scholar] [CrossRef]
- Gallenkamp, D.; Fürstner, A. Stereoselective Synthesis ofE,Z-Configured 1,3-Dienes by Ring-Closing Metathesis. Application to the Total Synthesis of Lactimidomycin. J. Am. Chem. Soc. 2011, 133, 9232–9235. [Google Scholar] [CrossRef]
- Bieniek, M.; Bujok, R.; Stepowska, H.; Jacobi, A.; Hagenkötter, R.; Arlt, D.; Jarzembska, K.N.; Makal, A.; Woźniak, K.; Grela, K. New air-stable ruthenium olefin metathesis precatalysts derived from bisphenol S. J. Organomet. Chem. 2006, 691, 5289–5297. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Davidson, F.; Dias, H.V.R.; Goerlich, J.R.; Khasnis, D.; Marshall, W.J.; Prakasha, T.K. An Air Stable Carbene and Mixed Carbene “Dimers. ” J. Am. Chem. Soc. 1997, 119, 12742–12749. [Google Scholar] [CrossRef]
- Kadyrov, R.; Rosiak, A.; Tarabocchia, J.; Szadkowska, A.; Bieniek, M.; Grela, K. New concepts in designing ruthenium-based second generation olefin metathesis catalysts and their application. In Catalysis of Organic Reactions; CRC Press: Boca Raton, FL, USA, 2008; pp. 217–222. [Google Scholar]
- Ginzburg, Y.; Lemcoff, N. Hoveyda-Type Olefin Metathesis Complexes. In Olefin Metathesis; Wiley: Hoboken, NJ, USA, 2014; pp. 437–451. [Google Scholar]
- Grela, K.; Harutyunyan, S.; Michrowska, A. A Highly Efficient Ruthenium Catalyst for Metathesis Reactions. Angew. Chem. Int. Ed. 2002, 41, 4038–4040. [Google Scholar] [CrossRef] [Green Version]
- Michrowska, A.; Bujok, R.; Harutyunyan, S.; Sashuk, V.; Dolgonos, G.; Grela, K.; Dolgonos, G.A. Nitro-Substituted Hoveyda−Grubbs Ruthenium Carbenes: Enhancement of Catalyst Activity through Electronic Activation. J. Am. Chem. Soc. 2004, 126, 9318–9325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grela, K. Ruthenium Complexes as (Pre)catalysts for Metathesis Reactions. U.S. Patent 6,867,303, 15 March 2005. [Google Scholar]
- Bieniek, M.; Michrowska, A.; Gułajski, Ł.; Grela, K. A Practical Larger Scale Preparation of Second-Generation Hoveyda-Type Catalysts. Organometallics 2007, 26, 1096–1099. [Google Scholar] [CrossRef]
- Zhan, Z.-Y.J. Recyclable Ruthenium Catalysts for Metathesis Reactions. U.S. Patent 7,632,772, 15 December 2009. [Google Scholar]
- Lindner, F.; Friedrich, S.; Hahn, F. Total Synthesis of Complex Biosynthetic Late-Stage Intermediates and Bioconversion by a Tailoring Enzyme from Jerangolid Biosynthesis. J. Org. Chem. 2018, 83, 14091–14101. [Google Scholar] [CrossRef] [PubMed]
- Stellfeld, T.; Bhatt, U.; Kalesse, M. Synthesis of the A,B,C-Ring System of Hexacyclinic Acid. Org. Lett. 2004, 6, 3889–3892. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Zeng, X.; Hao, M.-H.; Wei, X.; Yee, N.K.; Busacca, C.A.; Han, Z.; Farina, V.; Senanayake, C.H. RCM Macrocyclization Made Practical: An Efficient Synthesis of HCV Protease Inhibitor BILN. Org. Lett. 2008, 10, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Farina, V.; Shu, C.; Zeng, X.; Wei, X.; Han, Z.; Yee, N.K.; Senanayake, C.H. Second-Generation Process for the HCV Protease Inhibitor BILN 2061: A Greener Approach to Ru-Catalyzed Ring-Closing Metathesis†. Org. Process Res. Dev. 2009, 13, 250–254. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Chaturvedi, P.R.; Luesch, H. Process development and scale-up total synthesis of largazole, a potent class i histone deacetylase inhibitor. Org. Process Res. Dev. 2018, 22, 190–199. [Google Scholar] [CrossRef]
- Breen, C.P.; Parrish, C.; Shangguan, N.; Majumdar, S.; Murnen, H.; Jamison, T.F.; Bio, M.M. A scalable membrane pervaporation approach for continuous flow olefin metathesis. Org. Process Res. Dev. 2020. [Google Scholar] [CrossRef]
- Tracz, A.; Matczak, M.; Urbaniak, K.; Skowerski, K. Nitro-grela-type complexes containing iodides–robust and selective catalysts for olefin metathesis under challenging conditions. Beilstein J. Org. Chem. 2015, 11, 1823–1832. [Google Scholar] [CrossRef] [Green Version]
- Marx, V.M.; Sullivan, A.H.; Melaimi, M.; Virgil, S.C.; Keitz, B.K.; Weinberger, D.S.; Bertrand, G.; Grubbs, R.H. Cyclic alkyl amino carbene (caac) ruthenium complexes as remarkably active catalysts for ethenolysis. Angew. Chem. Int. Ed. 2015, 54, 1919–1923. [Google Scholar] [CrossRef] [Green Version]
- Gawin, R.; Tracz, A.; Chwalba, M.; Kozakiewicz, A.; Trzaskowski, B.; Skowerski, K. Cyclic Alkyl Amino Ruthenium Complexes—Efficient Catalysts for Macrocyclization and Acrylonitrile Cross Metathesis. ACS Catal. 2017, 7, 5443–5449. [Google Scholar] [CrossRef] [Green Version]
- Schmid, T.E.; Dumas, A.; Colombel-Rouen, S.; Crévisy, C.; Baslé, O.; Mauduit, M. From environmentally friendly reusable ionic-tagged ruthenium-based complexes to industrially relevant homogeneous catalysts: Toward a sustainable olefin metathesis. Synlett 2017, 28, 773–798. [Google Scholar]
- Bieniek, M.; Bujok, R.; Milewski, M.; Arlt, D.; Kajetanowicz, A.; Grela, K. Making the family portrait complete: Synthesis of electron withdrawing group activated Hoveyda-Grubbs catalysts bearing sulfone and ketone functionalities. J. Organomet. Chem. 2020, 918, 121276. [Google Scholar] [CrossRef]
- Bieniek, M.; Samojłowicz, C.; Sashuk, V.; Bujok, R.; Śledź, P.; Lugan, N.; Lavigne, G.; Arlt, D.; Grela, K. Rational design and evaluation of upgraded Grubbs/Hoveyda olefin metathesis catalysts: Polyfunctional benzylidene ethers on the test bench. Organometallics 2011, 30, 4144–4158. [Google Scholar] [CrossRef]
- Eivgi, O.; Sutar, R.L.; Reany, O.; Lemcoff, N.G. Bichromatic photosynthesis of coumarins by UV filter-enabled olefin metathesis. Adv. Synth. Catal. 2017, 359, 2352–2357. [Google Scholar] [CrossRef]
- Ivry, E.; Frenklah, A.; Ginzburg, Y.; Levin, E.; Goldberg, I.; Kozuch, S.; Lemcoff, N.G.; Tzur, E. Light- and thermal-activated olefin metathesis of hindered substrates. Organometallics 2018, 37, 176–181. [Google Scholar] [CrossRef]
- Tzur, E.; Szadkowska, A.; Ben-Asuly , A.; Makal, A.; Goldberg, I.; Woźniak, K.; Grela , K.; Lemcoff, N.G. Studies on electronic effects in O-, N- and S-chelated ruthenium olefin-metathesis catalysts. Chem.-A Eur. J. 2010, 16, 8726–8737. [Google Scholar] [CrossRef]
- Żukowska, K.; Szadkowska, A.; Pazio, A.E.; Woźniak, K.; Grela, K. Thermal switchability of N-chelating Hoveyda-type catalyst containing a secondary amine ligand. Organometallics 2012, 31, 462–469. [Google Scholar] [CrossRef]
- Gawin, A.; Pump, E.; Slugovc, C.; Kajetanowicz, A.; Grela, K. Ruthenium amide complexes—Synthesis and catalytic activity in olefin metathesis and in ring-opening polymerisation. Eur. J. Inorg. Chem. 2018, 2018, 1766–1774. [Google Scholar] [CrossRef]
- Monsaert, S.; Lozano Vila, A.; Drozdzak, R.; Van Der Voort, P.; Verpoort, F. Latent olefin metathesis catalysts. Chem. Soc. Rev. 2009, 38, 3360–3372. [Google Scholar] [CrossRef] [Green Version]
- Eivgi, O.; Lemcoff, N.G. Turning the light on: Recent developments in photoinduced olefin metathesis. Synthesis 2018, 50, 49–63. [Google Scholar]
- Luan, X.; Mariz, R.; Gatti, M.; Costabile, C.; Poater, A.; Cavallo, L.; Linden, A.; Dorta, R. Identification and characterization of a new family of catalytically highly active imidazolin-2-ylidenes. J. Am. Chem. Soc. 2008, 130, 6848–6858. [Google Scholar] [CrossRef] [PubMed]
- Vieille-Petit, L.; Luan, X.; Mariz, R.; Blumentritt, S.; Linden, A.; Dorta, R. A new class of stable, saturated N-heterocyclic carbenes with N-naphthyl substituents: Synthesis, dynamic behavior, and catalytic potential. Eur. J. Inorg. Chem. 2009, 2009, 1861–1870. [Google Scholar] [CrossRef]
- Vieille-Petit, L.; Clavier, H.; Linden, A.; Blumentritt, S.; Nolan, S.P.; Dorta, R. Ruthenium olefin metathesis catalysts with N-heterocyclic carbene ligands bearing N-naphthyl side chains. Organometallics 2010, 29, 775–788. [Google Scholar] [CrossRef]
- Winter, P.; Hiller, W.; Christmann, M. Access to Skipped Polyene Macrolides through Ring-Closing Metathesis: Total Synthesis of the RNA Polymerase Inhibitor Ripostatin B. Angew. Chem. Int. Ed. 2012, 51, 3396–3400. [Google Scholar] [CrossRef]
- Ritter, T.; Hejl, A.; Wenzel, A.G.; Funk, T.W.; Grubbs, R.H. A standard system of characterization for olefin metathesis catalysts. Organometallics 2006, 25, 5740–5745. [Google Scholar] [CrossRef] [Green Version]
- Garber, S.B.; Kingsbury, J.S.; Gray, B.L.; Hoveyda, A.H. Efficient and Recyclable Monomeric and Dendritic Ru-Based Metathesis Catalysts. J. Am. Chem. Soc. 2000, 122, 8168–8179. [Google Scholar] [CrossRef]
- Rivard, M.; Blechert, S. Effective and Inexpensive Acrylonitrile Cross-Metathesis: Utilisation of Grubbs II Precatalyst in the Presence of Copper(I) Chloride. Eur. J. Org. Chem. 2003, 2003, 2225–2228. [Google Scholar] [CrossRef]
- Vieille-Petit, L.; Luan, X.; Gatti, M.; Blumentritt, S.; Linden, A.; Clavier, H.; Nolan, S.P.; Dorta, R. Improving Grubbs’ II type ruthenium catalysts by appropriately modifying the N-heterocyclic carbene ligand. Chem. Commun. 2009, 25, 3783–3785. [Google Scholar] [CrossRef] [Green Version]
- Samojłowicz, C.; Bieniek, M.; Grela, K. Ruthenium-based olefin metathesis catalysts bearing N-heterocyclic carbene ligands. Chem. Rev. 2009, 109, 3708–3742. [Google Scholar] [CrossRef]
- Barbasiewicz, M.; Szadkowska, A.; Makal, A.; Jarzembska, K.N.; Grela, K.; Woźniak, K. Is the Hoveyda-Grubbs Complex a Vinylogous Fischer-Type Carbene? Aromaticity-Controlled Activity of Ruthenium Metathesis Catalysts. Chem.-A Eur. J. 2008, 14, 9330–9337. [Google Scholar] [CrossRef] [PubMed]
- Falivene, L.; Credendino, R.; Poater, A.; Petta, A.; Serra, L.; Oliva, R.; Scarano, V.; Cavallo, L. SambVca A Web Tool for Analyzing Catalytic Pockets with Topographic Steric Maps. Organometallics 2016, 35, 2286–2293. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.K.; Choi, T.-L.; Sanders, D.P.; Grubbs, R.H. A general model for selectivity in olefin cross metathesis. J. Am. Chem. Soc. 2003, 125, 11360–11370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, N.; Planer, S.; Grela, K. Formation of tetrasubstituted C–C double bonds via olefin metathesis: Challenges, catalysts, and applications in natural product synthesis. Org. Chem. Front. 2018, 5, 494–516. [Google Scholar] [CrossRef]
- Diver, S.T.; Griffiths, J.R. Ene-yne metathesis. In Olefin Metathesis: Theory and Practice; Grela, K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 153–185. [Google Scholar]
- Grotevendt, A.G.D.; Lummiss, J.A.M.; Mastronardi, M.L.; Fogg, D.E. Ethylene-Promoted versus Ethylene-Free Enyne Metathesis. J. Am. Chem. Soc. 2011, 133, 15918–15921. [Google Scholar] [CrossRef]
- Schmid, T.E.; Bantreil, X.; Citadelle, C.A.; Slawin, A.; Cazin, C.S.J. Phosphites as ligands in ruthenium-benzylidene catalysts for olefin metathesis. Chem. Commun. 2011, 47, 7060–7062. [Google Scholar] [CrossRef]
- Guidone, S.; Blondiaux, E.; Samojłowicz, C.; Gułajski, Ł.; Kędziorek, M.; Malinska, M.; Pazio, A.; Wozniak, K.; Grela, K.; Doppiu, A.; et al. Catalytic and Structural Studies of Hoveyda-Grubbs Type Pre-Catalysts Bearing Modified Ether Ligands. Adv. Synth. Catal. 2012, 354, 2734–2742. [Google Scholar] [CrossRef]
- Broggi, J.; Urbina-Blanco, C.A.; Clavier, H.; Leitgeb, A.; Slugovc, C.; Slawin, A.; Nolan, S.P. The Influence of Phosphane Ligands on the Versatility of Ruthenium-Indenylidene Complexes in Metathesis. Chem.-A Eur. J. 2010, 16, 9215–9225. [Google Scholar] [CrossRef]
- Lecourt, C.; Dhambri, S.; Allievi, L.; Sanogo, Y.; Zeghbib, N.; Ben Othman, R.; Lannou, M.-I.; Sorin, G.; Ardisson, J. Natural products and ring-closing metathesis: synthesis of sterically congested olefins. Nat. Prod. Rep. 2018, 35, 105–124. [Google Scholar] [CrossRef]
- Bieniek, M.; Michrowska, A.; Usanov, D.L.; Grela, K. In an attempt to provide a user’s guide to the galaxy of benzylidene, alkoxybenzylidene, and indenylidene ruthenium olefin metathesiss catalysts. Chem. A Eur. J. 2008, 14, 806–818. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
















| Catalyst | Solvent | Time (min) | Temp. (°C) | Yield (%) |
|---|---|---|---|---|
| 4a | Toluene | 60 | 80 | 83 |
| 4b | Toluene | 60 | 80 | 60 |
| 4c | Toluene | 60 | 60 | 63 |
| 4d | DCM | 20 | 40 | 87 |
| 4e | DCM | 10 | 40 | 77 |
| 4a [75] | 4b | 4c | 4d | 4e a | 4f [24] | |
|---|---|---|---|---|---|---|
| Ru‒C(1) | 1.979(3) | 1.985(4) | 1.990(4) | 1.970(3) | 2.002(9) 2.012(9) | 2.013(2) |
| Ru‒O(1) | 2.287(1) | 2.244(2) | 2.232(2) | 2.254(2) | 2.285(5) 2.252(5) | 2.310(2) |
| Ru‒C(2) | 1.825(2) | 1.829(4) | 1.821(3) | 1.827(3) | 1.836(9) 1.838(9) | 1.825(3) |
| Ru‒Cl(1) | 2.333(1) | 2.324(1) | 2.335(1) | 2.328(1) | 2.328(2) 2.328(2) | 2.343(1) |
| Ru‒Cl(2) | 2.330(1) | 2.333(1) | 2.339(1) | 2.331(1) | 2.328(2) 2.324(2) | 2.343(1) |
| C(1)-Ru-O(1) | 178.45(6) | 172.5(1) | 175.6(1) | 175.4(1) | 176.8(3) 177.8(3) | 175.93(8) |
| C(1)-Ru-C(2) | 101.36(8) | 102.1(1) | 103.0(1) | 102.4(1) | 99.9(4) 101.6(4) | 105.1(1) |
| Ru-C(2)-C(3)-C(4) | 8.6(2) | −8.9(5) | 5.9(4) | −5.4(3) | −2(1) 2(1) | −4.5(3) |
| C(2)-Ru-C(1)-N(1) | 7.6(2) | 14.2(4) | 5.5(4) | 13.0(3) | −31.8(9) 27(1) | 0.8(2) |
| α | 19.8(1) | 20.8(3) | 20.1(2) | 19.0(2) | 19.4(7) 12.4(7) | 25.4(2) |
| VBur (%) | 35.4 | 36.5 | 34.7 | 34.6 | 34.8 36.1 | 38.0 |
| Catalyst | Conversion (%) | |
|---|---|---|
| After 1 h | After 24 h | |
| 4a | 76 | 82 |
| 4b | 83 | 91 |
| 4c | 78 | 80 |
| 4d | 98 | 99 |
| 4e | 95 | 97 |
| Catalyst | Conversion (%) | |
|---|---|---|
| After 6 h | After 24 h | |
| 4a | 16 | 18 |
| 4b | 9 | 63 |
| 4c | 21 | 21 |
| 4d | 14 | 66 |
| 4e | 62 | 84 |
| Catalyst | Conversion (%) | |
|---|---|---|
| After 6 h | After 24 h | |
| 4a | 93 | 99 |
| 4b | 87 | 87 |
| 4c | 90 | 93 |
| 4d | 60 | 73 |
| 4e | 81 | 88 |
| Catalyst | Conversion (%) | |
|---|---|---|
| After 6 h | After 24 h | |
| 4a | 88 | 99 |
| 4b | 15 | 42 |
| 4c | 94 | 97 |
| 4d | 94 | 98 |
| 4e | 74 | 98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieczykolan, M.; Czaban-Jóźwiak, J.; Malinska, M.; Woźniak, K.; Dorta, R.; Rybicka, A.; Kajetanowicz, A.; Grela, K. The Influence of Various N-Heterocyclic Carbene Ligands on Activity of Nitro-Activated Olefin Metathesis Catalysts. Molecules 2020, 25, 2282. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25102282
Pieczykolan M, Czaban-Jóźwiak J, Malinska M, Woźniak K, Dorta R, Rybicka A, Kajetanowicz A, Grela K. The Influence of Various N-Heterocyclic Carbene Ligands on Activity of Nitro-Activated Olefin Metathesis Catalysts. Molecules. 2020; 25(10):2282. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25102282
Chicago/Turabian StylePieczykolan, Michał, Justyna Czaban-Jóźwiak, Maura Malinska, Krzysztof Woźniak, Reto Dorta, Anna Rybicka, Anna Kajetanowicz, and Karol Grela. 2020. "The Influence of Various N-Heterocyclic Carbene Ligands on Activity of Nitro-Activated Olefin Metathesis Catalysts" Molecules 25, no. 10: 2282. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25102282