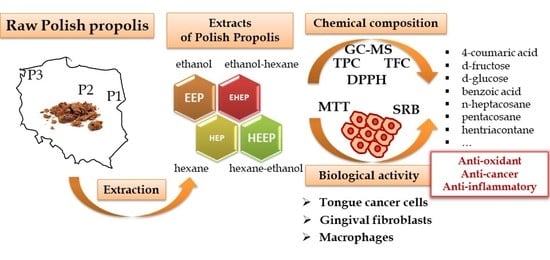
Polish Propolis—Chemical Composition and Biological Effects in Tongue Cancer Cells and Macrophages
Abstract
:1. Introduction
2. Results
2.1. Extraction Yield
2.2. Total Polyphenol Content
2.3. Total Flavonoid Content
2.4. GC-MS Analysis
Fatty Acids Composition
2.5. DPPH Free Radical Scavenging Activity
2.6. Anticancer Activity
2.6.1. MTT Assay
2.6.2. SRB Assay
2.7. Anti-Inflammatory Potential
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Extraction
4.3. Total Polyphenol Content
4.4. Total Flavonoid Content
4.5. GC-MS Analysis
Fatty Acids Composition
4.6. DPPH Free Radical Scavenging Activity
4.7. Biological Characterisation
4.7.1. Cell Culture
4.7.2. MTT Assay
4.7.3. SRB Assay
4.8. Statistical Analysis
5. Conclusions
- The highest total extraction yields were obtained for ethanol and hexane-ethanol extracts (EEP and HEEP);
- Total polyphenol content (TPC) and total flavonoid content (TFC) of ethanol and hexane-ethanol extracts (EEP and HEEP) were much higher than TPC and TFC of ethanol-hexane and hexane extracts (EHEP and HEP);
- Antioxidant potential of ethanol and hexane-ethanol extracts (EEP and HEEP) was much higher than that of ethanol-hexane and hexane extracts (EHEP and HEP);
- The extracts selected (EEP and HEEP) demonstrated anticancer activity in the tongue cancer cells; 24 h incubation affected cell viability and cellular proliferation significantly;
- The propolis extracts tested at higher concentrations (500 and 1000 µg/mL) impaired the proliferation of normal cells as well;
- The observed cytotoxicity of the extracts prepared towards murine macrophages requires further investigation to evaluate their possible anti-inflammatory potential.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Substances | RT | RI exp | RI lit | EEP_P1[%] | EEP_P2[%] | EEP_P3[%] | |
|---|---|---|---|---|---|---|---|
| 1 | Benzyl alcohol, TMS derivative | 6.110 | 1155 | 1152 | 0.12 | 0.15 | 0.1 |
| 2 | Benzoic Acid, TMS derivative | 8.795 | 1246 | 1249 | 4.35 | 3.8 | 3.15 |
| 3 | Glycerol, TMS | 10.162 | 1287 | 1289 | 0.78 | 0.99 | 1.55 |
| 4 | Butanedioic acid, 2TMS derivative | 11.445 | 1320 | 1321 | 0.12 | 0.04 | 0.13 |
| 5 | 1-Monoacetin, 2O-TMS | 11.740 | 1326 | 1324 | 0.13 | 0.07 | 0.08 |
| 6 | 4-Hydroxybenzaldehyde, TMS derivative | 13.737 | 1373 | 1383 | 0.22 | 0.16 | 0.08 |
| 7 | Hydroquinone, 2TMS derivative | 15.308 | 1409 | 1408 | 0.24 | 0.2 | 0.08 |
| 8 | Malic acid, 3TMS derivative | 20.278 | 1511 | 1497 | 0.09 | 0.06 | 1.1 |
| 9 | 5-Oxoproline, TMS derivative | 20.680 | 1521 | 1527 | 0.1 | 0.09 | - |
| 10 | Vanillin, TMS derivative | 21.622 | 1536 | 1530 | 1.48 | 0.5 | 0.37 |
| 11 | Cinnamic acid, TMS derivative | 22.048 | 1545 | 1542 | 0.12 | 0.14 | 0.25 |
| 12 | 4-Hydroxybenzoic acid, 2TMS derivative | 26.623 | 1634 | 1635 | 0.27 | 0.22 | 0.08 |
| 13 | Dodecanoic acid, TMS | 27.858 | 1658 | 1655 | 0.13 | 0.09 | - |
| 14 | β-D-Xylopyranose, 4TMS derivative | 34.330 | 1784 | 1777 | 0.17 | - | - |
| 15 | o-Coumaric acid, 2TMS derivative | 34.975 | 1797 | 1815 | 0.26 | 0.26 | 0.09 |
| 16 | D-Psicofuranose | 37.465 | 1848 | 1837 | 1.28 | 1.2 | 0.76 |
| 17 | D-Fructose, 5TMS derivative | 37.855 | 1856 | 1867 | 7.4 | 9.55 | 8.51 |
| 18 | D-Sorbitol, 6TMS derivative | 41.075 | 1922 | 1920 | 0.22 | 0.09 | 7.15 |
| 19 | D-Glucose, 5TMS derivative | 41.635 | 1934 | 1928 | 4.82 | 8.5 | - |
| 20 | 4-Coumaric acid, 2TMS derivative | 42.223 | 1947 | 1949 | 10.74 | 13.68 | 1.34 |
| 21 | D-Glucitol, 6TMS derivative | 43.902 | 1982 | 1980 | 0.44 | 0.34 | 0.18 |
| 22 | Gallic acid, 4TMS derivative | 44.118 | 1986 | 1987 | 0.39 | 0.1 | 0.21 |
| 23 | Salicylic acid, trimethylsilyl ether, benzyl ester | 45.910 | 2028 | 2025 | 0.55 | 0.49 | 0.11 |
| 24 | D-Mannopyranose, 5TMS derivative | 46.347 | 2038 | 2037 | 4.87 | 8.84 | 7.8 |
| 25 | D-Gluconic acid, 6TMS derivative | 46.875 | 2052 | 2043 | 0.16 | 0.16 | 0.09 |
| 26 | Palmitic Acid, TMS derivative | 47.070 | 2057 | 2050 | 0.85 | 0.7 | 0.69 |
| 27 | Isoferulic acid, 2TMS derivative | 48.435 | 2090 | 2081 | 0.17 | 0.67 | 2.7 |
| 28 | Ferulic acid, 2TMS derivative | 48.950 | 2103 | 2103 | 4.79 | 2.95 | 2.69 |
| 29 | Myo-Inositol, 6TMS derivative | 49.822 | 2132 | 2129 | 0.18 | 0.1 | 1.17 |
| 30 | Phtalic acid derivative* | 50.387 | 2150 | - | 1.07 | 1.19 | 4.02 |
| 31 | Caffeic acid, 3TMS derivative | 50.548 | 2157 | 2155 | 1.06 | 2.15 | - |
| 32 | Unknown | 51.908 | 2202 | - | 1.54 | 2.48 | 0.19 |
| 33 | 13-Octadecenoic acid, (E)-, TMS derivative | 52.383 | 2222 | 2228 | 0.59 | 0.72 | 0.88 |
| 34 | 3,7,11,15-Tetramethyl-2,6,10,14-hexadecatetraene-1-ol trimethylsilyl ether | 52.697 | 2236 | 2234 | 1.65 | 2.7 | 0.25 |
| 35 | Tricosane | 54.272 | 2300 | 2300 | 1.14 | 0.42 | 0.08 |
| 36 | Unknown | 55.733 | 2370 | - | - | - | 1.21 |
| 37 | Unknown | 56.810 | 2424 | - | - | - | 2.4 |
| 38 | Pterostilbene, trimethylsilyl ether | 57.507 | 2462 | - | 0.39 | 0.48 | 0.82 |
| 39 | Pentacosane | 58.223 | 2501 | 2506 | 0.83 | 0.26 | 0.64 |
| 40 | Unknown | 58.548 | 2519 | - | 4.41 | 4.32 | 1.5 |
| 41 | Ethyl trans-caffeate, bis(tert-butyldimethylsilyl) ether | 58.675 | 2527 | 2547 | 0.11 | 0.08 | 0.16 |
| 42 | Bisphenol C* | 59.085 | 2551 | - | 1.47 | 3.58 | 5.2 |
| 43 | 1-Docosanol, TMS derivative | 59.220 | 2558 | 2557 | 0.25 | 0.48 | 0.67 |
| 44 | Unknown | 59.577 | 2579 | - | 0.3 | 0.19 | 0.45 |
| 45 | Butanoic acid, 4-methoxy-2-nitro-, 2,6-bis(1,1-dimethylethyl)-4-methoxyphenyl ester | 59.990 | 2604 | 2595 | 0.6 | 0.25 | 3.25 |
| 46 | Unknown | 60.465 | 2635 | - | 0.45 | 0.71 | 0.56 |
| 47 | Behenic acid, TMS derivative | 60.650 | 2645 | 2644 | 1.05 | 0.37 | - |
| 48 | Unknown | 60.938 | 2664 | - | 0.82 | 1.22 | 2.59 |
| 49 | Unknown | 61.132 | 2675 | - | 0.27 | 0.59 | 1.51 |
| 50 | Unknown | 61.280 | 2685 | - | 2.12 | 0.96 | 0.9 |
| 51 | Maltose, 8TMS derivative, isomer 2 | 61.415 | 2693 | 2693 | 1.56 | 2.96 | 5.33 |
| 52 | n-Heptacosane | 61.527 | 2700 | 2700 | 0.82 | 0.2 | - |
| 53 | Sucrose, 8TMS derivative | 61.700 | 2712 | 2712 | 2.38 | 0.43 | 0.23 |
| 54 | D-(+)-Turanose, octakis(trimethylsilyl) ether, methyloxime (isomer 1) | 61.925 | 2727 | 2724 | 0.53 | 1.96 | 4.92 |
| 55 | Maltose, OTMS | 62.035 | 2735 | 2733 | 0.38 | 0.18 | 0.11 |
| 56 | D-Cellobiose, (isomer 2), 8TMS derivative | 62.258 | 2749 | 2762 | 1.7 | 2.64 | 0.43 |
| 57 | Naringenin, O,O’-bis(trimethylsilyl)- | 62.612 | 2772 | 2778 | 1.07 | 3.48 | 6.06 |
| 58 | Unknown | 63.180 | 2813 | - | - | - | 2.08 |
| 59 | Isosakuranetin, TMS derivative | 63.345 | 2821 | 2818 | 0.74 | 1.43 | 0.2 |
| 60 | Lignoceric acid, TMS derivative | 63.648 | 2842 | 2838 | 7.82 | 3.45 | 0.35 |
| 61 | Sakuranetin, TMS derivative | 64.200 | 2882 | 2877 | 0.96 | 0.58 | 0.26 |
| 62 | Catechine, 5TMS derivative | 64.872 | 2932 | 2938 | 0.17 | 0.13 | - |
| 63 | Gettibiose, TMS derivative | 65.712 | 2991 | 2989 | 0.39 | 0.38 | 0.29 |
| 64 | Triacontane | 65.933 | 3009 | 3003 | 0.44 | 0.36 | 0.27 |
| 65 | Pectolinaringenin, TMS derivative | 66.108 | 3021 | 3037 | 0.54 | 0.41 | 0.38 |
| 66 | Hexacosanoic acid, TMS derivative | 66.367 | 3041 | 3039 | 0.7 | 0.18 | 1.84 |
| 67 | Nonacosan-10-ol, O-TMS | 66.840 | 3078 | 3048 | 2.59 | 0.64 | - |
| 68 | Nonacosan-9-ol, O-TMS | 66.925 | 3085 | 3053 | 2.13 | 1.06 | 0.91 |
| 69 | Hentriacontane | 67.132 | 3100 | 3103 | 0.36 | 0.2 | 0.83 |
| 70 | Kaempferol, 4TMS | 67.298 | 3114 | 3112 | 0.29 | 0.86 | 0.77 |
| 71 | Trimethylsilyl octacosanoate, TMS derivative | 69.220 | 3256 | 3229 | 0.52 | 0.2 | 0.52 |
| 72 | Methyl triacontyl ether | 69.512 | 3275 | 3233 | 8.38 | 0.97 | 0.44 |
| Substances | RT | RI exp | RI lit | HEP_P1[%] | HEP_P2[%] | HEP_P3[%] | |
|---|---|---|---|---|---|---|---|
| 1 | Benzoic Acid, TMS derivative | 8.765 | 1246 | 1249 | 8.1 | 8.47 | 13.19 |
| 2 | Glycerol, TMS | 10.137 | 1287 | 1289 | 0.82 | 0.47 | 0.6 |
| 3 | Decanoic acid, TMS derivative | 17.830 | 1460 | 1450 | 0.15 | 1.5 | 0.14 |
| 4 | Vanillin, TMS derivative | 21.590 | 1536 | 1530 | 0.79 | 0.36 | 0.27 |
| 5 | Cinnamic acid, TMS derivative | 21.998 | 1545 | 1542 | 0.17 | 0.26 | 0.87 |
| 6 | Dodecanoic acid, TMS | 27.828 | 1658 | 1655 | 0.31 | 0.37 | 0.36 |
| 7 | β-D-Xylopyranose, 4TMS derivative | 34.300 | 1784 | 1777 | 0.25 | 1.36 | - |
| 8 | D-Fructose, 5TMS derivative | 37.770 | 1856 | 1867 | 0.36 | 0.36 | 0.38 |
| 9 | 4,7,10-Hexadecatrienoic acid, methyl ester | 40.008 | 1899 | 1902 | 0.36 | 0.13 | - |
| 10 | D-Sorbitol, 6TMS derivative | 41.032 | 1922 | 1920 | 0.2 | - | - |
| 11 | 4-Coumaric acid, 2TMS derivative | 42.122 | 1947 | 1949 | 0.33 | 0.19 | 0.21 |
| 12 | Salicylic acid, trimethylsilyl ether, benzyl ester | 45.865 | 2028 | 2025 | 0.87 | 1.84 | 0.63 |
| 13 | Palmitic Acid, TMS derivative | 47.035 | 2057 | 2050 | 2.48 | 3.06 | 2.96 |
| 14 | Ferulic acid, 2TMS derivative | 48.887 | 2103 | 2103 | 1.2 | 0.76 | 3.01 |
| 15 | Phtalic acid derivative* | 50.357 | 2150 | 1.04 | 1.31 | 0.21 | |
| 16 | Methyl caffeate, 2TMS derivative | 51.882 | 2201 | 1997 | 1.02 | 1.67 | 0.07 |
| 17 | 13-Octadecenoic acid, (E)-, TMS derivative | 52.365 | 2222 | 2228 | 1.7 | 4.3 | 8.05 |
| 18 | 3,7,11,15-Tetramethyl-2,6,10,14-hexadecatetraene-1-ol trimethylsilyl ether | 52.677 | 2236 | 2234 | 0.77 | 1.21 | - |
| 19 | Stearic acid, TMS derivative | 53.047 | 2249 | 2246 | 0.55 | 0.89 | 1.15 |
| 20 | Tricosane | 54.255 | 2300 | 2300 | 3.55 | 2.67 | 3.05 |
| 21 | Arachidic acid, TMS derivative | 57.212 | 2446 | 2447 | 0.43 | 0.4 | 0.63 |
| 22 | Pterostilbene, trimethylsilyl ether | 57.723 | 2462 | - | 0.34 | 0.28 | 0.13 |
| 23 | Pentacosane | 58.200 | 2501 | 2506 | 5.24 | 4.6 | 4.00 |
| 24 | Ethyl trans-caffeate, bis(tert-butyldimethylsilyl) ether | 58.518 | 2527 | 2547 | 0.84 | 1.54 | 1.2 |
| 25 | Bisphenol C* | 59.043 | 2551 | 0.3 | 0.52 | 1.28 | |
| 26 | Behenic acid, TMS derivative | 60.617 | 2643 | 2644 | 2.11 | 1.42 | 2.19 |
| 27 | Unknown | 61.255 | 2683 | - | 2.02 | 1.56 | 1.77 |
| 28 | n-Heptacosane | 61.512 | 2700 | 2700 | 14.11 | 14.31 | 12.45 |
| 29 | Maltose, OTMS | 62.007 | 2735 | 2733 | 0.38 | 0.19 | 0.1 |
| 30 | D-Cellobiose, (isomer 2), 8TMS derivative | 62.290 | 2749 | 2762 | 0.37 | 1.16 | 0.55 |
| 31 | Octacosane | 63.003 | 2798 | 2800 | 0.56 | 1.09 | 0.68 |
| 32 | Lignoceric acid, TMS derivative | 63.620 | 2842 | 2838 | 7.72 | 5.88 | 12.02 |
| 33 | Sakuranetin, TMS derivative | 64.170 | 2882 | 2877 | 0.53 | - | 0.23 |
| 34 | Nonacosane | 64.438 | 2899 | 2900 | 7.15 | 9.24 | 7.34 |
| 35 | Hexacosanoic acid, TMS derivative | 66.345 | 3041 | 3039 | 0.63 | 0.67 | 2.21 |
| 36 | Nonacosan-10-ol, O-TMS | 66.817 | 3078 | 3048 | 5.01 | 3.31 | 3.27 |
| 37 | Nonacosan-9-ol, O-TMS | 66.907 | 3085 | 3053 | 4.36 | 3.24 | 2.7 |
| 38 | Hentriacontane | 67.115 | 3100 | 3103 | 4.25 | 6.66 | 4.62 |
| 39 | Myristic acid, 9-hexadecenyl ester, (Z)- | 68.128 | 3177 | 3130 | 0.6 | 0.34 | 0.17 |
| 40 | Trimethylsilyl octacosanoate, TMS derivative | 69.188 | 3256 | 3229 | 1.21 | 1.01 | 0.5 |
| 41 | Methyl triacontyl ether | 69.493 | 3276 | 3233 | 16.8 | 11.36 | 6.82 |
| Substances | RT | RI exp | RI lit | HEEP_P1[%] | HEEP_P2[%] | HEEP_P3[%] | |
|---|---|---|---|---|---|---|---|
| 1 | Benzyl alcohol, TMS derivative | 6.112 | 1155 | 1152 | 0.09 | 0.04 | 0.08 |
| 2 | Benzoic Acid, TMS derivative | 8.797 | 1246 | 1249 | 3.16 | 1.24 | 1.27 |
| 3 | Cinnamaldehyde | 9.905 | 1272 | 1274 | 0.04 | 0.08 | 0.06 |
| 4 | Glycerol, TMS | 10.170 | 1287 | 1289 | 0.87 | 1.05 | 1.8 |
| 5 | Butanedioic acid, 2TMS derivative | 11.465 | 1320 | 1321 | 0.12 | 0.05 | 0.18 |
| 6 | 1-Monoacetin, 2O-TMS | 11.748 | 1326 | 1324 | 0.14 | 0.06 | 0.09 |
| 7 | 4-Hydroxybenzaldehyde, TMS derivative | 13.748 | 1373 | 1383 | 0.26 | 0.19 | 0.14 |
| 8 | Hydroquinone, 2TMS derivative | 15.325 | 1409 | 1408 | 0.4 | 0.22 | 0.13 |
| 9 | Cinnamyl alcohol, trimethylsilyl ether | 16.273 | 1422 | 1428 | - | 0.02 | 0.03 |
| 10 | Malic acid, 3TMS derivative | 20.282 | 1511 | 1497 | 0.12 | 0.12 | 0.81 |
| 11 | 5-Oxoproline, TMS derivative | 20.693 | 1521 | 1527 | 0.12 | 0.06 | 0.05 |
| 12 | Vanillin, TMS derivative | 21.640 | 1537 | 1530 | 1.82 | 0.46 | 0.27 |
| 13 | Cinnamic acid, TMS derivative | 22.043 | 1545 | 1542 | 0.07 | 0.05 | 0.15 |
| 14 | 3,4-Dihydroxybenzaldehyde, | 26.045 | 1622 | 1612 | - | 0.13 | 0.27 |
| 15 | 4-Hydroxybenzoic acid, 2TMS derivative | 26.638 | 1634 | 1635 | 0.48 | 0.22 | 0.18 |
| 16 | Dodecanoic acid, TMS | 27.668 | 1658 | 1655 | 0.14 | 0.02 | 0.08 |
| 17 | β-D-Xylopyranose, 4TMS derivative | 34.563 | 1784 | 1777 | 0.13 | 0.03 | 0.03 |
| 18 | o-Coumaric acid, 2TMS derivative | 34.968 | 1797 | 1815 | 0.28 | 0.25 | 0.09 |
| 19 | 4-Methoxycinnamic acid, TMS derivative | 36.602 | 1830 | 1833 | - | 0.08 | 0.26 |
| 20 | D-Psicofuranose | 37.475 | 1848 | 1837 | 1.46 | 1.59 | 2.24 |
| 21 | D-Fructose, 5TMS derivative | 37.865 | 1856 | 1866 | 12.58 | 11.92 | 2.03 |
| 22 | D-Sorbitol, 6TMS derivative | 41.092 | 1922 | 1920 | 0.16 | 0.1 | - |
| 23 | D-Glucose, 5TMS derivative | 41.658 | 1934 | 1928 | 7.14 | 11.9 | 8.27 |
| 24 | 4-Coumaric acid, 2TMS derivative | 42.252 | 1947 | 1949 | 16.74 | 13.52 | 10.74 |
| 25 | D-Glucitol, 6TMS derivative | 43.917 | 1982 | 1980 | 0.43 | 0.25 | 0.44 |
| 26 | Gallic acid, 4TMS derivative | 44.125 | 1987 | 1987 | 0.68 | 0.13 | - |
| 27 | Salicylic acid, trimethylsilyl ether, benzyl ester | 45.932 | 2028 | 2025 | 0.1 | 0.08 | 0.2 |
| 28 | D-Mannopyranose, 5TMS derivative | 46.353 | 2038 | 2037 | 6.33 | 13.13 | 9.13 |
| 29 | D-Gluconic acid, 6TMS derivative | 46.885 | 2052 | 2043 | 0.15 | 0.2 | 0.08 |
| 30 | Palmitic Acid, TMS derivative | 47.083 | 2057 | 2050 | 0.49 | 0.18 | 0.09 |
| 31 | Isoferulic acid, 2TMS derivative | 48.453 | 2090 | 2081 | 0.09 | 0.78 | 2.95 |
| 32 | Ferulic acid, 2TMS derivative | 48.965 | 2103 | 2103 | 7.93 | 3.02 | 3.86 |
| 33 | Myo-Inositol, 6TMS derivative | 49.845 | 2132 | 2129 | 0.09 | 0.13 | 1.3 |
| 34 | Phtalic acid derivative* | 50.403 | 2150 | - | 0.7 | 0.24 | 0.03 |
| 35 | Caffeic acid, 3TMS derivative | 50.565 | 2157 | 2155 | 0.89 | 0.83 | 0.03 |
| 36 | Linoleic acid, TMS | 51.922 | 2203 | 2212 | 1.31 | 2.48 | 4.6 |
| 37 | 13-Octadecenoic acid, (E)-, TMS derivative | 52.392 | 2222 | 2228 | 0.17 | 1.77 | 0.06 |
| 38 | 3,7,11,15-Tetramethyl-2,6,10,14-hexadecatetraene-1-ol trimethylsilyl ether | 52.703 | 2236 | 2234 | 1.01 | 0.07 | 0.07 |
| 39 | 2’,6’-Dihydroxy 4’-methoxydihydrochalcone, trimethylsilyl ether | 52.717 | 2418 | 2405 | - | 1.99 | 0.1 |
| 40 | Pterostilbene, trimethylsilyl ether | 57.520 | 2462 | - | 0.43 | 0.77 | 2.58 |
| 41 | Pentacosane | 58.563 | 2501 | 2506 | 5.85 | 0.44 | 0.87 |
| 42 | Ethyl trans-caffeate, bis(tert-butyldimethylsilyl) ether | 58.560 | 2527 | 2547 | - | 3.55 | 2.22 |
| 43 | Bisphenol* | 59.088 | 2551 | 1.65 | 0.13 | 0.12 | |
| 44 | 1-Docosanol, TMS derivative | 59.223 | 2558 | 2557 | 0.19 | 4.51 | 4.76 |
| 45 | Butanoic acid, 4-methoxy-2-nitro-, 2,6-bis(1,1-dimethylethyl)-4-methoxyphenyl ester | 59.998 | 2604 | 2595 | 0.72 | 0.32 | 0.67 |
| 46 | Unknown | 60.475 | 2635 | 0.99 | 0.84 | 3.44 | |
| 47 | Behenic acid, TMS derivative | 60.550 | 2645 | 2644 | 0.31 | 0.94 | 0.83 |
| 48 | Unknown | 60.947 | 2664 | 0.95 | 1.73 | 2.77 | |
| 49 | Unknown | 61.285 | 2685 | 3.22 | 0.69 | 1.67 | |
| 50 | Maltose, 8TMS derivative, isomer 2 | 61.422 | 2693 | 2693 | 1.83 | 3.42 | 5.26 |
| 51 | n-Heptacosane | 61.537 | 2700 | 2700 | 0.21 | 0.11 | 0.31 |
| 52 | Sucrose, 8TMS derivative | 61.705 | 2712 | 2712 | 5.88 | 0.52 | 2.61 |
| 53 | D-(+)-Turanose, octakis(trimethylsilyl) ether, methyloxime (isomer 1) | 61.937 | 2727 | 2724 | 0.71 | 2.66 | 4.98 |
| 54 | Maltose, OTMS | 62.040 | 2735 | 2733 | 0.29 | 0.13 | 0.12 |
| 55 | D-Cellobiose, (isomer 2), 8TMS derivative | 62.262 | 2749 | 2762 | 1.51 | 0.32 | 1.3 |
| 56 | Naringenin, O,O’-bis(trimethylsilyl)- | 62.617 | 2772 | 2778 | 1.14 | 4.1 | 5.95 |
| 57 | Isosakuranetin, TMS derivative | 63.352 | 2821 | 2818 | 0.94 | 1.37 | 2.13 |
| 58 | Lignoceric acid, TMS derivative | 63.650 | 2842 | 2838 | 1.22 | 1.68 | 0.44 |
| 59 | Sakuranetin, TMS derivative | 64.215 | 2882 | 2877 | 1.75 | 0.64 | 0.11 |
| 60 | Catechine, 5TMS derivative | 64.880 | 2932 | 2938 | 0.33 | 0.11 | 0.07 |
| 61 | Gettibiose, TMS derivative | 65.720 | 2991 | 2989 | 0.76 | 0.36 | 0.66 |
| 62 | Triacontane | 65.948 | 3009 | 3003 | 0.78 | 0.4 | 0.35 |
| 63 | Hexacosanoic acid, TMS derivative | 66.375 | 3041 | 3039 | 0.5 | 0.69 | 2.04 |
| 64 | Unknown | 66.975 | 3085 | - | 0.66 | 0.61 | 0.74 |
| 65 | Hentriacontane | 67.137 | 3100 | 3103 | 0.49 | 0.23 | 0.77 |
| Substances | RT | RI exp | RI lit | HEP_P1[%] | HEP_P2[%] | HEP_P3[%] | |
|---|---|---|---|---|---|---|---|
| 1 | Benzoic acid, methyl ester | 15.055 | 1623 | 1612 | 0.69 | 2.73 | 0.92 |
| 2 | Lauric acid, methyl ester | 18.820 | 1877 | 1804 | 1.85 | 2.32 | 1.28 |
| 3 | cis-9-Tetradecenoic acid, methyl ester | 25.410 | 2116 | 2026 | 5.16 | 1.94 | 2.30 |
| 4 | Pentadecanoic acid, methyl ester | 27.530 | 2194 | 2108 | 1.75 | 0.79 | 0.70 |
| 5 | Hexadecanoic acid, methyl ester | 29.590 | 2271 | 2208 | 31.17 | 27.63 | 20.54 |
| 6 | Heptadecanoic acid, methyl ester | 32.790 | 2666 | 2309 | 9.68 | 7.67 | 6.34 |
| 7 | Octadecanoic acid, methyl ester | 34.345 | 2394 | 2418 | 6.40 | 5.28 | 4.23 |
| 8 | Oleic acid, methyl ester | 35.050 | 2488 | 2434 | 18.18 | 25.30 | 25.90 |
| 9 | cis-11-Octadecenoic acid, methyl ester | 35.565 | 2512 | 2468 | 1.19 | 0.48 | 0.81 |
| 10 | Linolenic acid, methyl ester | 37.775 | 2625 | 2571 | 0.95 | 2.38 | 5.86 |
| 11 | Eicosanoic acid, methyl ester | 38.180 | 2651 | 2639 | 1.02 | 1.86 | 1.89 |
| 12 | Docosanoic acid, methyl ester | 40.790 | 2844 | 2835 | 4.70 | 5.71 | 5.00 |
| 13 | Me. C20:4n3; Eicosa-(8,11,14,17)-tetraenoate <methyl> | 41.060 | 2866 | 2865 | 2.48 | 2.37 | 2.75 |
| 14 | Tetracosanoic acid, methyl ester | 42.805 | 3067 | 3039 | 14.79 | 13.91 | 21.49 |
References
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [Green Version]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytotherapy Res. 2016, 30, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.; Baltazar, F.; Aguiar, C.A.A. Propolis: A Complex Natural Product with a Plethora of Biological Activities That Can Be Explored for Drug Development. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannopoulou, E.; De Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Więckiewicz, W.; Miernik, M.; Więckiewicz, M.; Morawiec, T. Does Propolis Help to Maintain Oral Health? Evid.-Based Complement. Altern. Med. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [Green Version]
- Grecka, K.; Kuś, P.M.; Okińczyc, P.; Worobo, R.; Walkusz, J.; Szweda, P. The Anti-Staphylococcal Potential of Ethanolic Polish Propolis Extracts. Molecules 2019, 24, 1732. [Google Scholar] [CrossRef] [Green Version]
- Pobiega, K.; Kraśniewska, K.; Derewiaka, D.; Gniewosz, M. Comparison of the antimicrobial activity of propolis extracts obtained by means of various extraction methods. J. Food Sci. Technol. 2019, 56, 5386–5395. [Google Scholar] [CrossRef] [Green Version]
- Piekarz, T.; Mertas, A.; Wiatrak, K.; Rój, R.; Kownacki, P.; Śmieszek-Wilczewska, J.; Kopczyńska, E.; Wrzoł, M.; Cisowska, M.; Szliszka, E.; et al. The Influence of Toothpaste Containing Australian Melaleuca alternifolia Oil and Ethanolic Extract of Polish Propolis on Oral Hygiene and Microbiome in Patients Requiring Conservative Procedures. Molecules 2017, 22, 1957. [Google Scholar] [CrossRef] [Green Version]
- Wieczynska, A.; Wezgowiec, J.; Wieckiewicz, W.; Czarny, A.; Kulbacka, J.; Nowakowska, D.; Gancarz, R.; Wilk, K.A. Antimicrobial Activity, Cytotoxicity and Total Phenolic Content of Different Extracts of Propolis from the West Pomeranian Region in Poland. Acta Pol. Pharm.-Drug Res. 2017, 74, 715–722. [Google Scholar]
- Wojtyczka, R.D.; Dziedzic, A.; Idzik, D.; Kępa, M.; Kubina, R.; Kabała-Dzik, A.; Smoleń-Dzirba, J.; Stojko, J.; Sajewicz, M.; Wąsik, T.J. Susceptibility of Staphylococcus aureus Clinical Isolates to Propolis Extract Alone or in Combination with Antimicrobial Drugs. Molecules 2013, 18, 9623–9640. [Google Scholar] [CrossRef] [Green Version]
- Borawska, M.; Naliwajko, S.; Moskwa, J.; Markiewicz-Żukowska, R.; Puścion-Jakubik, A.; Soroczyńska, J. Anti-proliferative and anti-migration effects of Polish propolis combined with Hypericum perforatum L. on glioblastoma multiforme cell line U87MG. BMC Complement. Altern. Med. 2016, 16, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popova, M.; Giannopoulou, E.; Skalicka-Woźniak, K.; Graikou, K.; Widelski, J.; Giannopoulou, E.; Kalofonos, H.P.; Sivolapenko, G.; Gaweł-Bęben, K.; Antosiewicz, B.; et al. Characterization and Biological Evaluation of Propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Sokół-Łętowska, A.; Kucharska, A.Z.; Jaworska, D.; Czuba, Z.; Krol, W. Ethanolic Extract of Polish Propolis: Chemical Composition and TRAIL-R2 Death Receptor Targeting Apoptotic Activity against Prostate Cancer Cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z. MicroRNA expression and its implications for diagnosis and therapy of tongue squamous cell carcinoma. J. Cell. Mol. Med. 2015, 20, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.C.; Cheung, K.-W.; Sze, D.M.-Y. The Immunomodulatory and Anticancer Properties of Propolis. Clin. Rev. Allergy Immunol. 2012, 44, 262–273. [Google Scholar] [CrossRef]
- Russo, A.; Cardile, V.; Sánchez, F.; Troncoso, N.; Vanella, A.; Garbarino, J. Chilean propolis: Antioxidant activity and antiproliferative action in human tumor cell lines. Life Sci. 2004, 76, 545–558. [Google Scholar] [CrossRef]
- Yen, C.-H.; Chiu, H.-F.; Wu, C.-H.; Lu, Y.-Y.; Han, Y.-C.; Shen, Y.; Venkatakrishnan, K.; Wang, C.-K. Beneficial efficacy of various propolis extracts and their digestive products by in vitro simulated gastrointestinal digestion. LWT 2017, 84, 281–289. [Google Scholar] [CrossRef]
- Salehi, M.; Motallebnejad, M.; Moghadamnia, A.A.; Seyemajidi, M.; Khanghah, S.N.; Ebrahimpour, A.; Molania, T. An Intervention Airing the Effect of Iranian Propolis on Epithelial Dysplasia of the Tongue: A Preliminary Study. J. Clin. Diagn. Res. 2017, 11, ZC67–ZC70. [Google Scholar] [CrossRef]
- Ribeiro, D.R.; Alves Ângela, V.F.; Dos Santos, E.P.; Padilha, F.F.; Gomes, M.Z.; Rabelo, A.S.; Cardoso, J.; Massarioli, A.P.; Alencar, S.M.; De Albuquerque-Júnior, R.L.C.; et al. Inhibition of DMBA-induced Oral Squamous Cells Carcinoma Growth by Brazilian Red Propolis in Rodent Model. Basic Clin. Pharmacol. Toxicol. 2015, 117, 85–95. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Don, M.-J.; Hung, P.-S.; Shen, Y.-C.; Lo, Y.-S.; Chang, K.-W.; Chen, C.-F.; Ho, L.-K. Cytotoxicity of phenolic acid phenethyl esters on oral cancer cells. Cancer Lett. 2005, 223, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-Y.; Jim, W.-T.; Su, L.-C.; Chung, C.-J.; Lin, C.-Y.; Huo, C.; Tseng, J.-C.; Huang, S.-H.; Lai, C.-J.; Chen, B.-C.; et al. Caffeic Acid Phenethyl Ester Is a Potential Therapeutic Agent for Oral Cancer. Int. J. Mol. Sci. 2015, 16, 10748–10766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, Y.-Y.; Lin, H.-P.; Huo, C.; Su, L.-C.; Yang, J.; Hsiao, P.-H.; Chiang, H.-C.; Chung, C.-J.; Wang, H.-D.; Chang, J.-Y.; et al. Caffeic Acid Phenethyl Ester Suppresses Proliferation and Survival of TW2.6 Human Oral Cancer Cells via Inhibition of Akt Signaling. Int. J. Mol. Sci. 2013, 14, 8801–8817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czyżewska, U.; Siemionow, K.; Zaręba, I.; Miltyk, W. Proapoptotic Activity of Propolis and Their Components on Human Tongue Squamous Cell Carcinoma Cell Line (CAL-27). PLOS ONE 2016, 11, e0157091. [Google Scholar] [CrossRef]
- Asgharpour, F.; Moghadamnia, A.A.; Zabihi, E.; Kazemi, S.; Namvar, A.E.; Gholinia, H.; Motallebnejad, M.; Nouri, H.R. Iranian propolis efficiently inhibits growth of oral streptococci and cancer cell lines. BMC Complement. Altern. Med. 2019, 19, 266–268. [Google Scholar] [CrossRef] [Green Version]
- Sawaya, A.C.; Cunha, I.B.D.S.; Marcucci, M.C. Analytical methods applied to diverse types of Brazilian propolis. Chem. Central J. 2011, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rassu, G.; Cossu, M.; Langasco, R.; Carta, A.; Cavalli, R.; Giunchedi, P.; Gavini, E. Propolis as lipid bioactive nano-carrier for topical nasal drug delivery. Colloids Surfaces B Biointerfaces 2015, 136, 908–917. [Google Scholar] [CrossRef]
- Kubilienė, L.; Laugaliene, V.; Pavilonis, A.; Maruska, A.; Majiene, D.; Barčauskaitė, K.; Kubilius, R.; Kasparaviciene, G.; Savickas, A. Alternative preparation of propolis extracts: Comparison of their composition and biological activities. BMC Complement. Altern. Med. 2015, 15, 156. [Google Scholar] [CrossRef] [Green Version]
- Maciejewicz, W. Isolation of Flavonoid Aglycones from Propolis by A Column Chromatography Method and Their Identification By Gc-Ms And Tlc Methods. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 1171–1179. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Rana, R.M.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Boil. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Sahinler, N.; Kaftanoglu, O. Natural product propolis: Chemical composition. Nat. Prod. Res. 2005, 19, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Roleira, F.M.F.; Da Silva, E.J.T.; Varela, C.; Costa, S.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Tyszka-Czochara, M.; Paśko, P.; Reczyński, W.; Szlósarczyk, M.; Bystrowska, B.; Opoka, W. Zinc and propolis reduces cytotoxicity and proliferation in skin fibroblast cell culture: Total polyphenol content and antioxidant capacity of propolis. Boil. Trace Element Res. 2014, 160, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szliszka, E.; Kucharska, A.Z.; Sokół-Łętowska, A.; Mertas, A.; Czuba, Z.; Krol, W. Chemical Composition and Anti-Inflammatory Effect of Ethanolic Extract of Brazilian Green Propolis on Activated J774A.1 Macrophages. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Silva, B.; Rosalen, P.L.; Alencar, S.M.; Mayer, M.P.A. Anti-inflammatory mechanisms of neovestitol from Brazilian red propolis in LPS-activated macrophages. J. Funct. Foods 2017, 36, 440–447. [Google Scholar] [CrossRef]
- Parashar, K.; Sood, S.; Mehaidli, A.; Curran, C.; Vegh, C.; Nguyen, C.; Pignanelli, C.; Zhang, Z.; Liang, G.; Wang, Y.; et al. Evaluating the Anti-cancer Efficacy of a Synthetic Curcumin Analog on Human Melanoma Cells and Its Interaction with Standard Chemotherapeutics. Molecules 2019, 24, 2483. [Google Scholar] [CrossRef] [Green Version]
- Tsirigotis-Maniecka, M.; Szyk-Warszynska, L.; Michna, A.; Warszyński, P.; Wilk, K.A. Colloidal characteristics and functionality of rationally designed esculin-loaded hydrogel microcapsules. J. Colloid Interface Sci. 2018, 530, 444–458. [Google Scholar] [CrossRef]
- Makhafola, T.J.; Elgorashi, E.; McGaw, L.J.; Verschaeve, L.; Jn, E. The correlation between antimutagenic activity and total phenolic content of extracts of 31 plant species with high antioxidant activity. BMC Complement. Altern. Med. 2016, 16, 490. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Dong, Y.; Du, H.; Shi, H.; Peng, Y.; Li, X. Antioxidant Compounds from Propolis Collected in Anhui, China. Molecules 2011, 16, 3444–3455. [Google Scholar] [CrossRef]
- Zali, A.G.; Ehsanzadeh, P.; Szumny, A.; Matkowski, A. Genotype-specific response of Foeniculum vulgare grain yield and essential oil composition to proline treatment under different irrigation conditions. Ind. Crop. Prod. 2018, 124, 177–185. [Google Scholar] [CrossRef]
- Dominiak, M.; Saczko, J. Method of Primary Culture of Human Fibroblasts for Autologous Augmentation. Patent No. PL209784B1, 31 October 2011. [Google Scholar]
- Houghton, P.; Fang, R.; Techatanawat, I.; Steventon, G.; Hylands, P.J.; Lee, C. The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods 2007, 42, 377–387. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the extracts of propolis prepared are available from the authors. |





| Symbol | Sequence of Solvents | Extraction Yield [%] | ||
|---|---|---|---|---|
| P1 | P2 | P3 | ||
| EEP | ethanol | 33.4 | 57.5 | 63.7 |
| EHEP | ethanol–hexane | 24.2 | 8.4 | 13.3 |
| HEP | hexane | 28.2 | 17.5 | 14.5 |
| HEEP | hexane–ethanol | 32.9 | 42.7 | 47.9 |
| Propolis Extract | P1 | P2 | P3 |
|---|---|---|---|
| Total Polyphenol Content [mg GAE/g] | |||
| EEP | 222.05 ± 14.29 | 259.63 ± 11.73 | 275.79 ± 13.42 |
| EHEP | 16.36 ± 1.12 | 19.60 ± 1.07 | 18.02 ± 1.09 |
| HEP | 20.45 ± 4.08 | 45.02 ± 7.22 | 38.84 ± 6.40 |
| HEEP | 249.92 ± 8.64 | 277.19 ± 14.28 | 308.92 ± 15.85 |
| Total Flavonoid Content [mg QE/g] | |||
| EEP | 18.76 ± 0.66 | 22.19 ± 0.44 | 19.79 ± 0.19 |
| EHEP | 11.10 ± 0.06 | 10.87 ± 0.03 | 12.99 ± 0.07 |
| HEP | 12.23 ± 0.21 | 13.49 ± 0.13 | 14.45 ± 0.19 |
| HEEP | 19.00 ± 0.57 | 22.46 ± 0.40 | 21.63 ± 0.25 |
| Propolis Extract | IC50 [µg/mL] | ||
|---|---|---|---|
| P1 | P2 | P3 | |
| EEP | 78.02 ± 4.86 | 55.07 ± 7.39 | 33.01 ± 2.73 |
| HEEP | 62.84 ± 14.59 | 60.72 ± 2.89 | 40.92 ± 7.55 |
| Symbol | Region of Origin | The Most Abundant Plants in the Region | Bee Species |
|---|---|---|---|
| P1 | Podlasie (Hajnowka) | spruce (Picea abies L.)—30%, pine (Pinus sylvestris L.)—27%, alder (Alnus glutinosa L.)—20%, sessile oak (Quercus petraea L.)—10%, silver birch (Betula pendula L.)—7% | Apis mellifera carnica x Apis mellifera caucasica |
| P2 | Mazovia (Ciechanow) | pine (Pinus sylvestris L.)—70%, alder (Alnus glutinosa L.)—10%, sessile oak (Quercus petraea L.)—10%, silver birch (Betula pendula L.)—7% | Apis mellifera carnica |
| P3 | West Pomerania (Miedzyzdroje) | pine (Pinus sylvestris L.)—75%, alder (Alnus glutinosa L.)—5%, beech (Fagus sylvatica L.)—5%, sessile oak (Quercus petraea L.)—5%, silver birch (Betula pendula L.)—4% | Apis mellifera mellifera |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wezgowiec, J.; Wieczynska, A.; Wieckiewicz, W.; Kulbacka, J.; Saczko, J.; Pachura, N.; Wieckiewicz, M.; Gancarz, R.; Wilk, K.A. Polish Propolis—Chemical Composition and Biological Effects in Tongue Cancer Cells and Macrophages. Molecules 2020, 25, 2426. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25102426
Wezgowiec J, Wieczynska A, Wieckiewicz W, Kulbacka J, Saczko J, Pachura N, Wieckiewicz M, Gancarz R, Wilk KA. Polish Propolis—Chemical Composition and Biological Effects in Tongue Cancer Cells and Macrophages. Molecules. 2020; 25(10):2426. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25102426
Chicago/Turabian StyleWezgowiec, Joanna, Anna Wieczynska, Wlodzimierz Wieckiewicz, Julita Kulbacka, Jolanta Saczko, Natalia Pachura, Mieszko Wieckiewicz, Roman Gancarz, and Kazimiera A. Wilk. 2020. "Polish Propolis—Chemical Composition and Biological Effects in Tongue Cancer Cells and Macrophages" Molecules 25, no. 10: 2426. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25102426