Exacerbated LPS/GalN-Induced Liver Injury in the Stress-Sensitive Wistar Kyoto Rat Is Associated with Changes in the Endocannabinoid System
Abstract
:1. Introduction
2. Results
2.1. WKY Rats Display Anxiety-Related Phenotype in the Open Field and Elevated Plus Maze
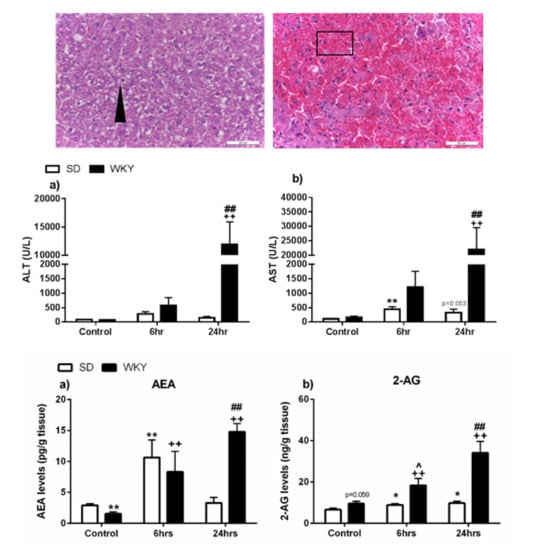
2.2. Histopathological Changes and Elevated Biochemical Markers of Liver Injury in WKY Rats in Response to LPS/GalN Compared to SD Counterparts
2.3. Altered Hepatic Inflammatory Profile Following LPS/GalN in WKY Compared with SD Rats
2.4. Alterations in the Hepatic Endocannabinoid System Prior to and Following LPS/GalN in WKY and SD Rats
2.5. Neither the Peripheral Restricted CB1 Receptor Antagonist AM6545 nor CB2 Receptor Agonist JWH-133 Altered LPS/GalN-Induced Liver Injury in SD or WKY Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.2.1. Assessment of LPS/GalN-Induced Inflammation, Liver Injury and Changes in Endocannabinoid System in SD and WKY Rats
4.2.2. The Effect of Systemic Administration of the Peripherally Restricted CB1 Antagonist AM6545 or CB2 Agonist JWH133 on LPS/GalN-Induced Inflammation and Liver Injury in SD and WKY Rats
4.3. Anxiety-Related Behaviour
4.4. Liver Histology
4.5. Plasma Biochemical Analysis
4.6. Gene Expression Analysis Using qRT-PCR
4.7. Enzyme-Linked Immunosorbent Assay (ELISA) for TNF-α and IL-6
4.8. Caspase-3 Activity Assay
4.9. Hepatic Glutathione Levels
4.10. Western Immunoblotting
4.11. Endocannabinoid Auantification Using Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-AG | 2-Arachidonoylglycerol |
| AEA | Anandamide |
| ALI | Acute liver injury |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| CB1 | Cannabinoid receptor 1 |
| CB2 | Cannabinoid receptor 2 |
| FAAH | Fatty acid amide hydrolase |
| GalN | d-Galactosamine |
| GLDH | Glutamate dehydrogenase |
| GSH | Glutathione |
| GSSG | Oxidised glutathione |
| IL | Interleukin |
| LPS | Lipopolysaccharide |
| MAGL | Monoacylglycerol lipase |
| SD | Sprague Dawley |
| MD2 | Myeloid adaptor protein 2 |
| TLR4 | Toll-like receptor 4 |
| WKY | Wistar-Kyoto |
References
- Zhan, Y.; Wang, Z.; Yang, P.; Wang, T.; Xia, L.; Zhou, M.; Wang, Y.; Wang, S.; Hua, Z.; Zhang, J. Adenosine 5′-monophosphate ameliorates D-galactosamine/lipopolysaccharide-induced liver injury through an adenosine receptor-independent mechanism in mice. Cell Death Dis. 2014, 5, e985. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-T. The role of the autonomic nervous system in chemically-induced liver damage and repair-using the essential hypertensive animal model (SHR). J. Auton. Nerv. Syst. 1995, 51, 135–142. [Google Scholar] [CrossRef]
- Felipo, V. Hepatic encephalopathy: Effects of liver failure on brain function. Nat. Rev. Neurosci 2013, 14, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Punzalan, C.S.; Barry, C.T. Acute Liver Failure: Diagnosis and Management. J. Intensive Care Med. 2016, 31, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, N.; Sugawara, Y.; Kokudo, N. Acute liver failure and liver transplantation. Intractable Rare Dis. Res. 2013, 2, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Galanos, C.; Freudenberg, M.A.; Reutter, W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc. Natl. Acad. Sci. USA 1979, 76, 5939–5943. [Google Scholar] [CrossRef] [Green Version]
- Sass, G.; Heinlein, S.; Agli, A.; Bang, R.; Schümann, J.; Tiegs, G. Cytokine Expression in Three Mouse Models Of Experimental Hepatitis. Cytokine 2002, 19, 115–120. [Google Scholar] [CrossRef]
- Ambade, A.; Catalano, D.; Lim, A.; Mandrekar, P. Inhibition of heat shock protein (molecular weight 90 kDa) attenuates proinflammatory cytokines and prevents lipopolysaccharide-induced liver injury in mice. Hepatology 2012, 55, 1585–1595. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.-M.; Zhang, J.-X.; Luo, J.; Guo, H.-X.; Deng, H.; Chen, J.-Y.; Sun, S.-L. A Role of Cell Apoptosis in Lipopolysaccharide (LPS)-induced Nonlethal Liver Injury in d-galactosamine (d-GalN)-sensitized Rats. Dig. Dis. Sci. 2007, 53, 1316–1324. [Google Scholar] [CrossRef]
- Vere, C.C.; Streba, C.T.; Streba, L.M.; Ionescu, A.G.; Sima, F. Psychosocial stress and liver disease status. World J. Gastroenterol. 2009, 15, 2980–2986. [Google Scholar] [CrossRef]
- Russ, T.C.; Kivimäki, M.; Morling, J.R.; Starr, J.M.; Stamatakis, E.; Batty, G.D. Association Between Psychological Distress and Liver Disease Mortality: A Meta-analysis of Individual Study Participants. Gastroenterology 2015, 148, 958–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, N.N.; Coppinger, J.; Deaver, D.R.; Roche, M.; Finn, D.P.; Kelly, J.P. Sex differences and similarities in depressive- and anxiety-like behaviour in the Wistar-Kyoto rat. Physiol. Behav. 2016, 167, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paré, W.P. The performance of WKY rats on three tests of emotional behavior. Physiol. Behav. 1992, 51, 1051–1056. [Google Scholar] [CrossRef]
- Burke, N.N.; Hayes, E.; Calpin, P.; Kerr, D.; Moriarty, O.; Finn, D.P.; Roche, M. Enhanced nociceptive responding in two rat models of depression is associated with alterations in monoamine levels in discrete brain regions. Neuroscience 2010, 171, 1300–1313. [Google Scholar] [CrossRef]
- Madasu, M.K.; Okine, B.N.; Olango, W.M.; Rea, K.; Lenihan, R.; Roche, M.; Finn, D.P. Genotype-dependent responsivity to inflammatory pain: A role for TRPV1 in the periaqueductal grey. Pharmacol. Res. 2016, 113, 44–54. [Google Scholar] [CrossRef]
- Kamisako, T.; Ogawa, H. Effect of obstructive jaundice on the regulation of hepatic cholesterol metabolism in the rat. Disappearance of abcg5 and abcg8 mRNA after bile duct ligation. Hepatol. Res. 2003, 25, 99–104. [Google Scholar] [CrossRef]
- Basu, P.P.; Aloysius, M.M.; Shah, N.J.; Brown, R.S., Jr. Review article: The endocannabinoid system in liver disease, a potential therapeutic target. Aliment. Pharmacol. Ther. 2014, 39, 790–801. [Google Scholar] [CrossRef]
- Tam, J.; Liu, J.; Mukhopadhyay, B.; Cinar, R.; Godlewski, G.; Kunos, G. Endocannabinoids in liver disease. Hepatology 2011, 53, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Mallat, A.; Teixeira-Clerc, F.; Lotersztajn, S. Cannabinoid signaling and liver therapeutics. J. Hepatol. 2013, 59, 891–896. [Google Scholar] [CrossRef] [Green Version]
- Melgar-Lesmes, P.; Perramón, M.; Jiménez, W. Roles of the Hepatic Endocannabinoid and Apelin Systems in the Pathogenesis of Liver Fibrosis. Cells 2019, 8, 1311. [Google Scholar] [CrossRef] [Green Version]
- Bazwinsky, I.; Zipprich, A.; Dehghani, F. Endocannabinoid System in Hepatic Glucose Metabolism, Fatty Liver Disease, and Cirrhosis. Int. J. Mol. Sci. 2019, 20, 2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, R.J.; Kerr, D.M.; Finn, D.P.; Roche, M. For whom the endocannabinoid tolls: Modulation of innate immune function and implications for psychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Caraceni, P.; Viola, A.; Piscitelli, F.; Giannone, F.; Berzigotti, A.; Cescon, M.; Domenicali, M.; Petrosino, S.; Giampalma, E.; Riili, A.; et al. Circulating and hepatic endocannabinoids and endocannabinoid-related molecules in patients with cirrhosis. Liver Int. 2009, 30, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Clerc, F.; Julien, B.; Grenard, P.; Van Nhieu, J.T.; Deveaux, V.; Li, L.; Serriere-Lanneau, V.; Ledent, C.; Mallat, A.; Lotersztajn, S. CB1 cannabinoid receptor antagonism: A new strategy for the treatment of liver fibrosis. Nat. Med. 2006, 12, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Rivera, P.; Vargas, A.; Pastor, A.; Boronat, A.; López-Gambero, A.J.; Sánchez-Marín, L.; Medina-Vera, D.; Serrano, A.; Pavón, F.J.; De La Torre, R.; et al. Differential hepatoprotective role of the cannabinoid CB 1 and CB 2 receptors in paracetamol-induced liver injury. Br. J. Pharmacol. 2020, 177, 3309–3326. [Google Scholar] [CrossRef] [PubMed]
- Julien, B.; Grenard, P.; Teixeira-Clerc, F.; Van Nhieu, J.T.; Li, L.; Karsak, M.; Zimmer, A.; Mallat, A.; Lotersztajn, S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 2005, 128, 742–755. [Google Scholar] [CrossRef]
- Dibba, P.; Li, A.A.; Cholankeril, G.; Iqbal, U.; Gadiparthi, C.; Khan, M.A.; Kim, D.; Ahmed, A. The Role of Cannabinoids in the Setting of Cirrhosis. Medicines 2018, 5, 52. [Google Scholar] [CrossRef] [Green Version]
- Mendez-Sanchez, N.; Zamora-Valdés, D.; Pichardo-Bahena, R.; Barredo-Prieto, B.; Ponciano-Rodriguez, G.; Bermejo-Martínez, L.; Chávez-Tapia, N.C.; Baptista-González, H.A.; Uribe, M. Endocannabinoid receptor CB2 in nonalcoholic fatty liver disease. Liver Int. 2007, 27, 215–219. [Google Scholar] [CrossRef]
- Louvet, A.; Teixeira-Clerc, F.; Chobert, M.-N.; Deveaux, V.; Pavoine, C.; Zimmer, A.; Pecker, F.; Mallat, A.; Lotersztajn, S. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology 2011, 54, 1217–1226. [Google Scholar] [CrossRef]
- Leist, M.; Gantner, F.; Bohlinger, I.; Tiegs, G.; Germann, P.G.; Wendel, A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am. J. Pathol. 1995, 146, 1220–1234. [Google Scholar]
- Jaeschke, H.; Fisher, M.A.; Lawson, J.A.; Simmons, C.A.; Farhood, A.; Jones, D.A. Activation of Caspase 3 (CPP32)-Like Proteases Is Essential for TNF-α-Induced Hepatic Parenchymal Cell Apoptosis and Neutrophil-Mediated Necrosis in a Murine Endotoxin Shock Model. J. Immunol. 1998, 160, 3480–3486. [Google Scholar] [PubMed]
- Chen, Y.; Dong, H.; Thompson, D.; Shertzer, H.; Nebert, D.; Vasiliou, V. Glutathione defense mechanism in liver injury: Insights from animal models. Food Chem. Toxicol. 2013, 60, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiegs, G.; Wolter, M.; Wendel, A. Tumor necrosis factor is a terminal mediator in galactosamine/endotoxin-induced hepatitis in mice. Biochem. Pharmacol. 1989, 38, 627–631. [Google Scholar] [CrossRef]
- McCoy, K.L. Interaction between Cannabinoid System and Toll-Like Receptors Controls Inflammation. Mediat. Inflamm. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Vinod, K.Y.; Xie, S.; Psychoyos, D.; Hungund, B.L.; Cooper, T.B.; Tejani-Butt, S.M. Dysfunction in Fatty Acid Amide Hydrolase Is Associated with Depressive-Like Behavior in Wistar Kyoto Rats. PLoS ONE 2012, 7, e36743. [Google Scholar] [CrossRef] [Green Version]
- Rea, K.; Olango, W.M.; Okine, B.N.; Madasu, M.K.; McGuire, I.C.; Coyle, K.; Harhen, B.; Roche, M.; Finn, D.P. Impaired endocannabinoid signalling in the rostral ventromedial medulla underpins genotype-dependent hyper-responsivity to noxious stimuli. Pain 2014, 155, 69–79. [Google Scholar] [CrossRef]
- Smaga, I.; Jastrzębska, J.; Zaniewska, M.; Bystrowska, B.; Gawliński, D.; Faron-Górecka, A.; Broniowska, Ż.; Miszkiel, J.; Filip, M. Changes in the Brain Endocannabinoid System in Rat Models of Depression. Neurotox. Res. 2017, 31, 421–435. [Google Scholar] [CrossRef] [Green Version]
- Shoval, G.; Shbiro, L.; Hershkovitz, L.; Hazut, N.; Zalsman, G.; Mechoulam, R.; Weller, A. Prohedonic Effect of Cannabidiol in a Rat Model of Depression. Neuropsychobiology 2016, 73, 123–129. [Google Scholar] [CrossRef]
- Hen-Shoval, D.; Amar, S.; Shbiro, L.; Smoum, R.; Haj, C.G.; Mechoulam, R.; Zalsman, G.; Weller, A.; Shoval, G. Acute oral cannabidiolic acid methyl ester reduces depression-like behavior in two genetic animal models of depression. Behav. Brain Res. 2018, 351, 1–3. [Google Scholar] [CrossRef]
- Cao, Z.; Mulvihill, M.M.; Mukhopadhyay, P.; Xu, H.; Erdélyi, K.; Hao, E.; Holovac, E.; Haskó, G.; Cravatt, B.F.; Nomura, D.K.; et al. Monoacylglycerol lipase controls endocannabinoid and eicosanoid signaling and hepatic injury in mice. Gastroenterology 2013, 144, 808–817. [Google Scholar] [CrossRef] [Green Version]
- Siegmund, S.V.; Qian, T.; De Minicis, S.; Harvey-White, J.; Kunos, G.; Vinod, K.Y.; Hungund, B.; Schwabe, R.F. The endocannabinoid 2-arachidonoyl glycerol induces death of hepatic stellate cells via mitochondrial reactive oxygen species. FASEB J. 2007, 21, 2798–2806. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, S.V.; Uchinami, H.; Osawa, Y.; Brenner, D.A.; Schwabe, R.F. Anandamide induces necrosis in primary hepatic stellate cells. Hepatology 2005, 41, 1085–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Kong, X.; Zhang, T.; Ye, J.; Fang, Z.; Yang, X. Pseudoephedrine/ephedrine shows potent anti-inflammatory activity against TNF-α-mediated acute liver failure induced by lipopolysaccharide/d-galactosamine. Eur. J. Pharmacol. 2014, 724, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Vemuri, V.K.; Liu, J.; Bátkai, S.; Mukhopadhyay, B.; Godlewski, G.; Osei-Hyiaman, D.; Ohnuma, S.; Ambudkar, S.V.; Pickel, J.; et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J. Clin. Investig. 2010, 120, 2953. [Google Scholar] [CrossRef] [Green Version]
- Cluny, N.; Vemuri, V.; Chambers, A.; Limebeer, C.; Bedard, H.; Wood, J.; Lutz, B.; Zimmer, A.; Parker, L.; Makriyannis, A.; et al. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br. J. Pharmacol. 2010, 161, 629–642. [Google Scholar] [CrossRef] [Green Version]
- Kimball, E.S.; Schneider, C.R.; Wallace, N.H.; Hornby, P.J. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am. J. Physiol. Liver Physiol. 2006, 291, G364–G371. [Google Scholar] [CrossRef] [Green Version]
- Singh, U.P.; Singh, N.P.; Singh, B.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Cannabinoids Receptor-2 (CB2) agonist ameliorates colitis in IL-10(−/−) mice by attenuating the activation of T cells and promoting their apoptosis. Toxicol. Appl. Pharmacol. 2012, 258, 256–267. [Google Scholar] [CrossRef] [Green Version]
- Teixeira-Clerc, F.; Belot, M.-P.; Manin, S.; Deveaux, V.; Cadoudal, T.; Chobert, M.-N.; Louvet, A.; Zimmer, A.; Tordjmann, T.; Mallat, A.; et al. Beneficial paracrine effects of cannabinoid receptor 2 on liver injury and regeneration. Hepatology 2010, 52, 1046–1059. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Ren, F.; Zhang, H.; Wen, T.; Piao, Z.; Zhou, L.; Zheng, S.; Zhang, J.; Chen, Y.; Han, Y.; et al. Inhibition of Glycogen Synthase Kinase 3β Ameliorates D-GalN/LPS-Induced Liver Injury by Reducing Endoplasmic Reticulum Stress-Triggered Apoptosis. PLoS ONE 2012, 7, e45202. [Google Scholar] [CrossRef] [Green Version]
- Flannery, L.E.; Henry, R.J.; Kerr, D.M.; Finn, D.P.; Roche, M. FAAH, but not MAGL, inhibition modulates acute TLR3-induced neuroimmune signaling in the rat, independent of sex. J. Neurosci. Res. 2017, 96, 989–1001. [Google Scholar] [CrossRef] [Green Version]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Killilea, M.; Kerr, D.M.; Mallard, B.M.; Roche, M.; Wheatley, A.M. Exacerbated LPS/GalN-Induced Liver Injury in the Stress-Sensitive Wistar Kyoto Rat Is Associated with Changes in the Endocannabinoid System. Molecules 2020, 25, 3834. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25173834
Killilea M, Kerr DM, Mallard BM, Roche M, Wheatley AM. Exacerbated LPS/GalN-Induced Liver Injury in the Stress-Sensitive Wistar Kyoto Rat Is Associated with Changes in the Endocannabinoid System. Molecules. 2020; 25(17):3834. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25173834
Chicago/Turabian StyleKillilea, Marykate, Daniel M. Kerr, Beth M. Mallard, Michelle Roche, and Antony M. Wheatley. 2020. "Exacerbated LPS/GalN-Induced Liver Injury in the Stress-Sensitive Wistar Kyoto Rat Is Associated with Changes in the Endocannabinoid System" Molecules 25, no. 17: 3834. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25173834