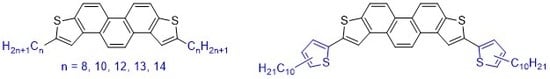
Synthesis and Physicochemical Properties of 2,7-Disubstituted Phenanthro[2,1-b:7,8-b’]dithiophenes
Abstract
:1. Introduction
2. Results and Discussion
3. Summary
4. Experimental Sections
4.1. General
4.2. Synthesis of 2,7-dibrominated PDT-2 1
4.3. General Procedure for the Palladium-Catalyzed Suzuki-Miyaura Coupling of 1 with Alkylboranes
4.4. General Procedure for the Palladium-Catalyzed Migita-Kosugi-Stille Coupling of 1 with (decylthiophene-2-yl)Tributylstannane
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsumura, A.; Koezuka, H.; Ando, T. Macromolecular electronic device: Field-effect transistor with a polythiophene thin film. Appl. Phys. Lett. 1986, 49, 1210–1212. [Google Scholar] [CrossRef]
- Sirringhaus, H. 25th Anniversary Article: Organic Field-Effect Transistors: The Path Beyond Amorphous Silicon. Adv. Mater. 2014, 26, 1319–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Dong, H.; Hu, W.; Liu, Y.; Zhu, D. Semiconducting π-conjugated systems in field-effect transistors: A material odyssey of organic electronics. Chem. Rev. 2012, 112, 2208–2267. [Google Scholar] [CrossRef] [PubMed]
- Takimiya, K.; Shinamura, S.; Osaka, I.; Miyazaki, E. Thienoacene-based organic semiconductors. Adv. Mater. 2011, 23, 4347–4370. [Google Scholar] [CrossRef]
- Mitsuhashi, R.; Suzuki, Y.; Yamanari, Y.; Mitamura, H.; Kambe, T.; Ikeda, N.; Okamoto, H.; Fujiwara, A.; Yamaji, M.; Kawasaki, N.; et al. Superconductivity in alkali-metal-doped picene. Nature 2010, 464, 76–79. [Google Scholar] [CrossRef]
- Facchetti, A. Semiconductors for organic transistors. Mater. Today 2007, 10, 28–37. [Google Scholar] [CrossRef]
- Minemawari, H.; Yamada, T.; Matsui, H.; Tsutsumi, J.; Haas, S.; Chiba, R.; Kumai, R.; Hasegawa, T. Inkjet printing of single-crystal films. Nature 2011, 475, 364–367. [Google Scholar] [CrossRef]
- Takeya, J.; Yamagishi, M.; Tominari, Y.; Hirahara, R.; Nakazawa, Y. Very high-mobility organic single-crystal transistors with in-crystal conduction channels. Appl. Phys. Lett. 2007, 90, 102120. [Google Scholar] [CrossRef]
- Watanabe, M.; Chen, K.-Y.; Chang, Y.J.; Chow, T.J. Acenes generated from precursors and their semiconducting properties. Acc. Chem. Res. 2013, 46, 1606–1615. [Google Scholar] [CrossRef]
- Kubozono, Y.; He, X.; Hamao, S.; Teranishi, K.; Goto, H.; Eguchi, R.; Kambe, T.; Gohda, S.; Nishihara, Y. Transistor application of phenacene molecules and their characteristics. Eur. J. Inorg. Chem. 2014, 24, 3806–3819. [Google Scholar] [CrossRef]
- Watanabe, M.; Chang, Y.J.; Liu, S.-W.; Chao, T.-H.; Goto, K.; Islam, M.M.; Yuan, C.-H.; Tao, Y.-T.; Shinmyozu, T.; Chow, T.J. The synthesis, crystal structure and charge-transport properties of hexacene. Nat. Chem. 2012, 4, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, C.; Tsuyama, H.; Shikata, R.; Murata, Y.; Kuniyasu, H.; Yamagishi, M.; Ishii, H.; Yamamoto, A.; Hirose, Y.; Yano, M.; et al. High performance solution-crystallized thin-film transistors based on V-shaped thieno[3,2-f:4,5-f’]bis[1]benzothiophene semiconductors. J. Mater. Chem. C 2017, 5, 1903–1909. [Google Scholar] [CrossRef]
- Ie, Y.; Ueta, M.; Nitani, M.; Tohnai, N.; Miyata, M.; Tada, H.; Aso, Y. Air-stable n-type organic field-effect transistors based on 4,9-dihydro-s-indaceno[1,2-b:5,6-b′]dithiazole-4,9-dione unit. Chem. Mater. 2012, 24, 3285–3293. [Google Scholar] [CrossRef]
- Mori, T.; Nishimura, T.; Yamamoto, T.; Doi, I.; Miyazaki, E.; Osaka, I.; Takimiya, K. Consecutive thiophene-annulation approach to π-extended thienoacene-based organic semiconductors with [1]benzothieno[3,2-b][1]benzothiophene (BTBT) substructure. J. Am. Chem. Soc. 2013, 135, 13900–13913. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, C.; Okamoto, T.; Yamagishi, M.; Tsurumi, J.; Yoshimoto, K.; Nakahara, K.; Soeda, J.; Hirose, Y.; Sato, H.; Yamano, A.; et al. High-performance solution-processable n-shaped organic semiconducting materials with stabilized crystal phase. Adv. Mater. 2014, 26, 4546–4551. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Yang, Y.S.; Matsuoka, K.; Yasuda, T. Effects of chalcogen atom substitution on the optoelectronic and charge-transport properties in picene-type π-systems. Chem. Commun. 2017, 53, 3814–3817. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Tu, Z.; Zhao, G.; Zhen, Y.; Geng, H.; Yi, Y.; Wang, Z.; Zhang, H.; Xu, C.; Liu, J.; et al. Tuning the crystal polymorphs of alkyl thienoacene via solution self-assembly toward air-stable and high-performance organic field-effect transistors. Adv. Mater. 2015, 27, 825–830. [Google Scholar] [CrossRef]
- Sawamoto, M.; Kang, M.J.; Miyazaki, E.; Sugino, H.; Osaka, I.; Takimiya, K. Soluble dinaphtho[2,3-b:2′,3′-f]thieno[3,2-b]thiophene derivatives for solution-processed organic field-effect transistors. ACS Appl. Mater. Interfaces 2016, 8, 3810–3824. [Google Scholar] [CrossRef] [Green Version]
- Back, J.Y.; An, T.K.; Cheon, Y.R.; Cha, H.; Jang, J.; Kim, Y.; Baek, Y.; Chung, D.S.; Kwon, S.-K.; Park, C.E.; et al. Alkyl chain length dependence of the field-effect mobility in novel anthracene derivatives. ACS Appl. Mater. Interfaces 2015, 7, 351–358. [Google Scholar] [CrossRef]
- Goetz, K.P.; Sekine, K.; Paulus, F.; Zhong, Y.; Roth, D.; Becker-Koch, D.; Hofstetter, Y.J.; Michel, E.; Reichert, L.; Rominger, F.; et al. The effect of side-chain length on the microstructure and processing window of zone-cast naphthalene-based bispentalenes. J. Mater. Chem. C 2019, 7, 13493–13501. [Google Scholar] [CrossRef]
- Ishida, T.; Sawanaka, Y.; Toyama, R.; Ji, Z.; Mori, H.; Nishihara, Y. Synthesis of dinaphtho[2,3-d:2′,3′-d’]anthra[1,2-b:5,6-b’]dithiophene (DNADT) derivatives: Effect of alkyl chains on transistor properties. Int. J. Mol. Sci. 2020, 21, 2447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.-C.; Nishinaga, S.; Okuda, Y.; Zhao, J.-J.; Xu, J.; Mori, H.; Nishihara, Y. A divergent synthesis of 3,10-dialkylpicenes. Org. Chem. Front. 2015, 2, 536–541. [Google Scholar] [CrossRef]
- Mori, H.; Chen, X.-C.; Chang, N.-H.; Hamao, S.; Kubozono, Y.; Nakajima, K.; Nishihara, Y. Synthesis of methoxy-substituted picenes: Substitution position effect on their electronic and single-crystal structures. J. Org. Chem. 2014, 79, 4973–4983. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Hamao, S.; Goto, H.; Sakai, Y.; Izumi, M.; Gohda, S.; Kubozono, Y.; Eguchi, R. Transistor application of alkyl-substituted picene. Sci. Rep. 2015, 4, 5048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, N.-H.; Chen, X.-C.; Nonobe, H.; Okuda, Y.; Mori, H.; Nakajima, K.; Nishihara, Y. Synthesis of substituted picenes through Pd-catalyzed cross-coupling reaction/annulation sequences and their physicochemical properties. Org. Lett. 2013, 15, 3558–3561. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Wang, J.-Y.; Pei, J. Roles of flexible chains in organic semiconducting materials. Chem. Mater. 2014, 26, 594–603. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kojiguchi, Y.; Kawata, S.; Mori, T.; Okamoto, K.; Tsutsui, M.; Koganezawa, T.; Katagiri, H.; Yasuda, T. Solution-processable organic semiconductors featuring S-shaped dinaphthothienothiophene (S-DNTT): Effects of alkyl chain length on self-organization and carrier transport properties. Chem. Mater. 2020, 32, 5350–5360. [Google Scholar] [CrossRef]
- Ma, Z.; Geng, H.; Wang, D.; Shuai, Z. Influence of alkyl side-chain length on the carrier mobility in organic semiconductors: Herringbone vs. pi-pi stacking. J. Mater. Chem. C 2016, 4, 4546–4555. [Google Scholar] [CrossRef]
- Minemawari, H.; Tanaka, M.; Tsuzuki, S.; Inoue, S.; Yamada, T.; Kumai, R.; Shimoi, Y.; Hasegawa, T. Enhanced layered-herringbone packing due to long alkyl chain substitution in solution-processable organic semiconductors. Chem. Mater. 2017, 29, 1245–1254. [Google Scholar] [CrossRef]
- Burnett, E.K.; Ai, Q.; Cherniawski, B.P.; Parkin, S.R.; Risko, C.; Briseno, A.L. Even−odd alkyl chain-length alternation regulates oligothiophene crystal structure. Chem. Mater. 2019, 31, 6900–6907. [Google Scholar] [CrossRef]
- Kawabata, K.; Usui, S.; Takimiya, K. Synthesis of soluble dinaphtho[2,3-b:2′,3′-f]thieno[3,2-b]thiophene (DNTT) derivatives: One-step functionalization of 2-bromo-DNTT. J. Org. Chem. 2020, 85, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Miyata, K.; Sugimoto, T.; Watanabe, K.; Uemura, T.; Takeya, J.; Matsumoto, Y. Enhancement of the exciton coherence size in organic semiconductor by alkyl chain substitution. J. Phys. Chem. C 2016, 120, 7941–7948. [Google Scholar] [CrossRef]
- Nishinaga, S.; Mori, H.; Nishihara, Y. Synthesis and transistor application of bis[1]benzothieno[6,7-d:6′,7′-d′]benzo[1,2-b:4,5-b′]dithiophenes. J. Org. Chem. 2018, 83, 5506–5515. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, K.; Nishinaga, S.; Sawanaka, Y.; Ishida, T.; Mori, H.; Nishihara, Y. Synthesis and physicochemical properties of dibenzo[2,3-d:2′,3′-d′]anthra[1,2-b:5,6-b′]dithiophene (DBADT) and its derivatives: Effect of substituents on their molecular orientation and transistor properties. J. Org. Chem. 2019, 84, 698–709. [Google Scholar] [CrossRef]
- Nishinaga, S.; Mitani, M.; Mori, H.; Okamoto, T.; Takeya, J.; Nishihara, Y. Bis[1]benzothieno[5,4-d:5′,4′-d’]benzo[1,2-b:4,5-b’]dithiophene derivatives: Synthesis and effect of sulfur positions on their transistor properties. Bull. Chem. Soc. Jpn. 2019, 92, 1107–1116. [Google Scholar] [CrossRef]
- Hyodo, K.; Hagiwara, H.; Toyama, R.; Mori, H.; Soga, S.-I.; Nishihara, Y. Bis[1]benzothieno[2,3-d:2′,3′-d’]anthra[1,2-b:5,6-b’]dithiophene: Synthesis, characterization, and application to organic field-effect transistors. RSC Adv. 2017, 7, 6089–6092. [Google Scholar] [CrossRef] [Green Version]
- Nishinaga, S.; Sawanaka, Y.; Toyama, R.; Ishida, T.; Mori, H.; Nishihara, Y. Synthesis and transistor characteristics of dinaphtho[2,3-d:2′,3′-d’]anthra[1,2-b:5,6-b’]dithiophene (DNADT). Chem. Lett. 2018, 47, 1409–1411. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Mitsudo, K.; Mandai, H.; Wakamiya, A.; Murata, Y.; Mori, H.; Nishihara, Y.; Suga, S. Efficient synthesis and properties of [1]benzothieno[3,2-b]thieno[2,3-d]furans and [1]benzothieno[3,2-b]thieno[2,3-d]thiophenes. Asian J. Org. Chem. 2018, 7, 1635–1641. [Google Scholar] [CrossRef]
- Oyama, T.; Mori, T.; Hashimoto, T.; Kamiya, M.; Ichikawa, T.; Komiyama, H.; Yang, Y.S.; Yasuda, T. High-mobility regioisomeric thieno[f,f′]bis[1]benzothiophenes: Remarkable effect of syn/anti thiophene configuration on optoelectronic properties, self-organization, and charge-transport functions in organic transistors. Adv. Electron. Mater. 2017, 4, 1700390. [Google Scholar] [CrossRef]
- Mori, T.; Oyama, T.; Komiyama, H.; Yasuda, T. Solution-grown unidirectionally oriented crystalline thin films of a U-shaped thienoacene-based semiconductor for high-performance organic field-effect transistors. J. Mater. Chem. C 2017, 5, 5872–5876. [Google Scholar] [CrossRef]
- Hyodo, K.; Toyama, R.; Mori, H.; Nishihara, Y. Synthesis and physicochemical properties of piceno[4,3-b:9,10-b′]dithiophene derivatives and their application in organic field-effect transistors. ACS Omega 2017, 2, 308–315. [Google Scholar] [CrossRef]
- Nishihara, Y.; Kinoshita, M.; Hyodo, K.; Okuda, Y.; Eguchi, R.; Goto, H.; Hamao, S.; Takabayashi, Y.; Kubozono, Y. Phenanthro[1,2-b:8,7-b’]dithiophene: A new picene- type molecule for transistor applications. RSC Adv. 2013, 3, 19341–19347. [Google Scholar] [CrossRef]
- Ebata, H.; Izawa, T.; Miyazaki, E.; Takimiya, K.; Ikeda, M.; Kuwabara, H.; Yui, T. Highly soluble [1]benzothieno[3,2-b]benzothiophene (BTBT) derivatives for high-performance, solution-processed organic field-effect transistors. J. Am. Chem. Soc. 2007, 129, 15732–15733. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Takimiya, K. Facile synthesis of highly π-extended heteroarenes, dinaphtho[2,3-b:2′,3′-f]chalcogenopheno[3,2-b]chalcogenophenes, and their application to field-effect transistors. J. Am. Chem. Soc. 2007, 129, 2224–2225. [Google Scholar] [CrossRef] [PubMed]
- Schweicher, G.; Lemaur, V.; Niebel, C.; Ruzié, C.; Diao, Y.; Goto, O.; Lee, W.-Y.; Kim, Y.; Arlin, J.-B.; Karpinska, J.; et al. Bulky end-capped [1]benzothieno[3,2-b]benzothiophenes: Reaching high-mobility organic semiconductors by fine tuning of the crystalline solid-state order. Adv. Mater. 2015, 27, 3066–3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, A.; Murata, Y.; Mitsui, C.; Ishii, H.; Yamagishi, M.; Yano, M.; Sato, H.; Yamano, A.; Takeya, J.; Okamoto, T. Zigzag-elongated fused π-electronic core: A molecular design strategy to maximize charge-carrier mobility. Adv. Sci. 2018, 5, 1700317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.-P.; Li, X.-Y.; Luo, X.-E.; Fei, X.; Sun, B.; Chen, L.-C.; Shi, Z.-F.; Sun, C.-L.; Shao, X.; Zhang, H.-L. Boosting the charge transport property of indeno[1,2-b]fluorene-6,12-dione though incorporation of sulfur- or nitrogen-linked side chains. Adv. Funct. Mater. 2017, 27, 1702318. [Google Scholar] [CrossRef]
- Hyodo, K.; Nonobe, H.; Nishinaga, S.; Nishihara, Y. Synthesis of 2,9-dialkylated phenanthro[1,2-b:8,7-b’]dithiophenes via cross-coupling reactions and sequential Lewis acid-catalyzed regioselective cycloaromatization of epoxide. Tetrahedron Lett. 2014, 55, 4002–4005. [Google Scholar] [CrossRef]
- Kubozono, Y.; Hyodo, K.; Mori, H.; Hamao, S.; Goto, H.; Nishihara, Y. Transistor application of new picene-type molecules, 2,9-dialkylated phenanthro[1,2-b:8,7-b’]dithiophenes. J. Mater. Chem. C 2015, 3, 2413–2421. [Google Scholar] [CrossRef]
- Kubozono, Y.; Hyodo, K.; Hamao, S.; Shimo, Y.; Mori, H.; Nishihara, Y. Transistor properties of 2,7-dialkyl-substituted phenanthro[2,1-b:7,8-b′]dithiophene. Sci. Rep. 2016, 6, 38535. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Kuroda, Y.; Ishii, H.; Yoshino, S.; Kobayashi, N. Second highest occupied molecular orbital effects on the valence band structure of organic semiconductors. Jpn. J. Appl. Phys. 2019, 58, SIIB27. [Google Scholar] [CrossRef]
- Inoue, S.; Minemawari, H.; Tsutsumi, J.; Chikamatsu, M.; Yamada, T.; Horiuchi, S.; Tanaka, M.; Kumai, R.; Yoneya, M.; Hasegawa, T. Effects of substituted alkyl chain length on solution-processable layered organic semiconductor crystals. Chem. Mater. 2015, 27, 3809–3812. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |





| Solution | Thin Film | |||||
|---|---|---|---|---|---|---|
| Compounds | λmaxabs (nm) | λmaxem (nm) | λedge (nm) | Egopt (eV) | Stokes shift (cm−1) | λmaxabs (nm) |
| PDT-2 | 251, 271, 302, 318, 333 | 364, 382, 402 | 366 | 3.39 | 305 | 282, 292 |
| C8-PDT-2 | 257, 276, 304, 324, 339 | 369, 386, 406 | 370 | 3.35 | 297 | 263, 287, 296, 349 |
| C10-PDT-2 | 257, 276, 304, 324, 339 | 369, 386, 406 | 370 | 3.35 | 297 | 263, 288, 297, 349 |
| C12-PDT-2 | 257, 276, 304, 324, 340 | 369, 387, 406 | 370 | 3.35 | 297 | 263, 289, 297, 350 |
| C13-PDT-2 | 257, 276, 304, 324, 339 | 369, 386, 406 | 370 | 3.35 | 297 | 263, 289, 300,351 |
| C14-PDT-2 | 256, 276, 304, 324, 339 | 369, 387, 407 | 369 | 3.36 | 297 | 262, 302, 335, 352, 371 |
| Th1-PDT-2 | 263, 293, 365, 384 | 399, 422 | 404 | 3.07 | 979 | 258, 323, 389 |
| Th2-PDT-2 | 263, 292, 362, 380 | 396, 418 | 397 | 3.12 | 1063 | 244, 319, 368, 389 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Z.; Cheng, Z.; Mori, H.; Nishihara, Y. Synthesis and Physicochemical Properties of 2,7-Disubstituted Phenanthro[2,1-b:7,8-b’]dithiophenes. Molecules 2020, 25, 3842. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25173842
Ji Z, Cheng Z, Mori H, Nishihara Y. Synthesis and Physicochemical Properties of 2,7-Disubstituted Phenanthro[2,1-b:7,8-b’]dithiophenes. Molecules. 2020; 25(17):3842. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25173842
Chicago/Turabian StyleJi, Zhenfei, Zeliang Cheng, Hiroki Mori, and Yasushi Nishihara. 2020. "Synthesis and Physicochemical Properties of 2,7-Disubstituted Phenanthro[2,1-b:7,8-b’]dithiophenes" Molecules 25, no. 17: 3842. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25173842