With the exception of the salan ligands and their Pd complexes, all chemicals and solvents were high-quality commercial products purchased from Sigma-Aldrich/Merck, St. Louis, Missouri, USA; VVR International, West Chester, Pennsylvania, USA; and Molar Chemicals Kft., Halásztelek, Hungary and were used without further purification. Good quality ion-exchanged water was used throughout (S ≤ 2 μS). Gases (Ar and N2) were supplied by Linde Gáz Magyarország Zrt., Répcelak, Hungary.
3.1. Synthesis of the Sulfosalan Ligands
HSS [
44], PrHSS [
45], BuHSS [
46] and
rac-CyHSS [
46] were synthesized according to published methods. Synthetic procedures for dPhHSS as well as for
cis- and
trans-CyHSS are described below.
3.1.1. 1,2-Diphenyl-N,N’-bis(2-hydroxy-5-sulfonatobenzyl)-1,2-diaminoethane-dPhHSS
This was prepared from the appropriate salen derivative (dPhS) by hydrogenation to afford the benzylamino intermediate (dPhHS) followed by sulfonation to yield dPhHSS.
Synthesis of dPhS:
meso-1,2-Diphenyl-ethylenediamine (4.0 g, 18.80 mol) was added into a round-bottom flask containing 50 mL ethanol. To this solution, salicylaldehyde (3.70 mL, 37.60 mmol) was added and the mixture was stirred at 25 °C for 1 h, resulting in formation of a yellow precipitate. The reaction mixture was filtered, and the product was washed with ethanol to obtain dPhS as a yellow crystalline solid. Yield was 7.58 g (17.93 mmol), 95%, yellow crystalline solid.
1H-NMR (d6-DMSO, 360 MHz, δ): 5.06 (s, 2H, –CH–CH–), 6.85 (d, J = 8.0 Hz, 4H, CHarom), 7.20–7.32 (m, 12H, CHarom), 8.43 (s, 2H, CH=N–), 13.17 (s, 2H, –OH).
13C{1H} NMR (d6-DMSO, 90 MHz, δ): 166.17, 160.11, 139.93, 132.53, 131.75, 128.23, 127.86, 127.44, 118.69, 118.50, 116.34, 77.70.
Synthesis of dPhHS
dPhS (4.00 g, 6.87 mmol) was dissolved in methanol (300 mL) followed by the addition of 4 equivalents (1.04 g, 27.48 mmol) of sodium borohydride in 100 mL of methanol under constant stirring at room temperature. The mixture was then stirred at reflux for 30 min. The hot reaction mixture was added dropwise into 600 mL of water with continuous stirring. The white precipitate was filtered, washed with water and dried under vacuum. Yield was 3.90 g (6.67 mmol), 97%, white solid.
1H-NMR (d6-DMSO, 360 MHz, δ): 3.00 (d, 14.0 Hz, 2H, CH2–NH) and 3.11 (d, 14.0 Hz, CH2–NH), 3.49 (s, 2H, –CH–CH–), 6.28–6.32 (m, 4H, CHarom), 6.46 (d, J = 7.2 Hz, 2H, CHarom), 6.68 (t, J = 7.3 Hz, 2H, CHarom), 6.94–7.04 (m, 10H, CHarom).
13C{1H} NMR (d6-DMSO, 90 MHz, δ: 156.51, 140.81, 128.41, 128.04, 127.98, 127.65, 127.13, 124.60, 118.32, 115.07, 66.82, 47.75.
Synthesis of dPhHSS
In a round-bottom flask, dPhHS (1.00 g, 2.34 mmol) was added in small portions to a mixture of 4 mL of 20% fuming sulfuric acid (oleum) and 1 mL of concentrated sulfuric acid. The flask was cooled in ice water, and the mixture was stirred for 60 min. Then, the content of the flask was carefully added to 25 mL of cold water. The pH of the reaction mixture was set to 4 with a concentrated NaOH solution. Then, the mixture was cooled for 24 h, during which a white precipitate formed. The solid was collected by filtration, washed with cold water and dried under vacuum. The compound thus obtained is the zwitterionic free acid form of the ligand, which is slightly soluble in water.
Yield was 825.05 mg (1.41 mmol), 60%, white solid.
1H-NMR (D2O, 360 MHz, δ): 3.14 (d, J = 14.0 Hz, 2H, CH2–NH), 3.27 (d, J = 14.0 Hz, 2H, CH2–NH), 3.89 (s, 2H, –CH–CH–), 6.43 (d, J = 8.4 Hz, 2H, CHarom), 7.05 (s, 2H, CHarom), 7.36–7.54 (m, 12H, CHarom).
13C{1H} NMR (D2O, 90 MHz, δ): 169.22, 139.50, 129.10, 128.28, 127.26, 126.64, 126.29, 125.56, 118.33, 66.02, 46.27.
IR (ATR), ν/cm−1: 594.7, 697.8, 759.0, 1033.6, 1101.7, 1181.0, 1285.9, 1590.8.
ESI-MS for C28H28N2O8S2 (m/z): calcd for [M − H]− 583.121, found 583.121.
3.1.2. N,N’-bis(2-Hydroxy-5-sulfonatobenzyl)-cis-1,2-diaminocyclohexane-cis-CyHSS
This was prepared from the appropriate salen derivative (cis-CyS) by hydrogenation to afford the benzylamino intermediate (cis-CyHS) followed by sulfonation to yield cis-CyHSS.
Synthesis of cis-CyS
According to
Section 3.1.1, 1.05 mL (8.76 mmol) of
cis-1,2-diaminocyclohexane and 1.83 mL (17.51 mmol) of salicylaldehyde yielded 2.50 g (7.71 mmol), 88%, yellow crystalline solid.
1H-NMR (d6-DMSO, 360 MHz, δ): 1.54–1.87 (m, 8H, –CH2–CH2–), 3.67 (s, 2H, CH–CH), 6.82–6.9 (m, 4H, CHarom), 7.31 (t, J = 7.4 Hz, 2H, CHarom), 7.41 (d, J = 7.3, 2H, CHarom), 8.56 (s, 2H, –CH=N–), 13.66 (s, 2H, –OH).
13C{1H} NMR (d6-DMSO, 90 MHz, δ): 165.05, 160.76, 132.25, 131.72, 118.66, 118.42, 116.45, 68.12, 30.42, 21.95.
Synthesis of cis-CyHS
According to
Section 3.1.1, 2.50 g (7.71 mmol) of
cis-CyS and 1.17 g (30.84 mmol) of sodium borohydride yielded 2.23 g (6.79 mmol), 88%, white crystalline solid.
1H-NMR (d6-DMSO, 360 MHz, δ): 1.25–1.36 (m, 4H, –CH2–CH2–), 1.54–1.65 (m, 4H, –CH2–CH2–), 2.72 (s, 2H, –CH–CH–), 3.69 (d, J = 13.9 Hz, CH2–NH), 3.78 (d, J = 13.9 Hz, CH2–NH), 6.72 (s, 2H, CHarom), 7.07 (d, J = 7. 6 Hz, 2H, CHarom).
13C{1H} NMR (d6-DMSO, 90 MHz, δ): 157.02, 128.74, 127.79, 125.07, 118.50, 115.30, 55.32, 47.58, 27.11, 21.97.
Synthesis of cis-CyHSS
According to
Section 3.1.1, 1 g (3.06 mmol) CyHS, 4 mL of 20% fuming sulfuric acid (oleum) and 1 mL of concentrated sulfuric acid yielded 821 mg (1.68 mmol), 55%, white crystalline solid.
1H-NMR (D2O, 360 MHz, δ): 1.33–1.69 (m, 8H, –CH2–CH2–), 2.83 (s, 2H, –CH–CH–), 3.59 (d, J = 13 Hz, CH2–NH), 3.69 (d, J = 13 Hz, CH2–NH), 6.60 (d, J = 8.7 Hz, 2H, CHarom), 7.43–7.47 (m, 4H, CHarom).
13C{1H} NMR (D2O, 90 MHz, δ): 169.31, 127.79, 127.39, 126.46, 126.18, 118.57, 55.69, 46.69, 26.81, 21.81.
IR (ATR), ν/cm−1: 588.8, 6923.0, 846.2, 1039.3, 1101.6, 1138,2, 1435.8, 1604.7.
ESI-MS for C20H26N2O8S2 (m/z): calcd for [M + H]+ 487.120, found 487.122 and [M + Na+]+ 509.102, found 509.104.
3.1.3. N,N’-bis(2-Hydroxy-5-sulfonatobenzyl)-trans-1,2-diaminocyclohexane - trans-CyHSS
This was prepared from the appropriate salen derivative (trans-CyS) by hydrogenation to afford the benzylamino intermediate (trans-CyHS) followed by sulfonation to yield trans-CyHSS.
Synthesis of trans-CyS
According to
Section 3.1.1, 3.06 mL (25.50 mmol) of
trans-1,2-diaminocyclohexane and 5.0 mL (51.00 mmol) of salicylaldehyde yielded 7.89 g (24.32 mmol), 95%, yellow crystalline solid.
1H-NMR (d6-DMSO, 360 MHz, δ): 1.39–1.45 (m, 2H, –CH2–CH2–), 1.59–1.62 (m, 2H, –CH2–CH2–), 1.76–1.88 (m, 4H, –CH2–CH2–), 3.36 (s, 2H, CH–CH), 6.81 (d, J = 8.0 Hz, 4H, CHarom), 7.24–7.29 (t, J = 8.1 Hz, 2H, CHarom), 7.32–7.35 (d, J = 7.3, 2H, CHarom), 8.47 (s, 2H, –CH=N–), 13.32 (s, 2H, –OH).
13C{1H} NMR (d6-DMSO, 90 MHz, δ): 165.00, 160.33, 132.16, 131.54, 118.48, 116.29, 71.29, 32.48, 23.67.
Synthesis of trans-CyHS
According to
Section 3.1.1, 7.90 g (24.04 mmol) of
trans-CyS and 3.64 g (61.65 mmol) of sodium borohydride yielded 7.11 g (21.65 mmol), 90%, white crystalline solid.
1H-NMR (d6-DMSO, 360 MHz, δ): 1.08–1.23 (m, 4H, –CH2–CH2–), 1.67 (s, 2H, –CH2–), 2.07–2.10 (m, 2H, –CH2–), 2.51 (s, 2H, –CH–CH–), 3.79 (d, J = 13.7 Hz, 2H, CH2–NH), 3.92 (d, J = 13.7 Hz, 2H, CH2–NH), 6.74–6.78 (t, J = 7.2 Hz, 2H, CHarom), 6.86 (d, J = 7.9 Hz, 2H, CHarom), 7.08–7.12 (t, J = 7.7 Hz, 2H, CHarom), 7.24 (d, J = 7.2 Hz, 2H, CHarom).
13C{1H} NMR (d6-DMSO, 90 MHz, δ): 156.04, 129.63, 128.53, 123.28, 118.80, 115.15, 58.91, 44.95, 28.73, 24.01.
Synthesis of trans-CyHSS
According to
Section 3.1.1, 1 g (3.06 mmol)
trans-CyHS, 4 mL of 20% fuming sulfuric acid (oleum) and 1 mL of concentrated sulfuric acid yielded 868 mg (1.78 mmol), 58%, white crystalline solid.
1H-NMR (D2O, 360 MHz, δ): 1.05–1.17 (m, 4H, –CH2–CH2–), 1.57–1.60 (m, 2H, –CH2–), 1.89–1.92 (m, 2H, –CH2–), 2.34–2.37 (m, 2H, –CH2–NH–), 3.59–3.67 (m, 4H, CH2–NH), 6.55 (d, J = 8.1 Hz, 2H, CHarom), 7.38–7.45 (m, 4H, CHarom).
13C{1H} NMR (D2O, 90 MHz), δ: 169.13, 128.13, 127.18, 126.34, 126.10, 118.51, 59.47, 46.48, 29.68, 24.06.
IR (ATR), ν/cm−1: 587.5, 694.4, 840.3, 1033.3, 1165.4, 1207.3, 1281.3, 1598.1
ESI-MS for C20H26N2O8S2 (m/z): calcd for [M + H]+ 487.119, found 487.121 and [M + Na+]+ 509.102, found 509.104.
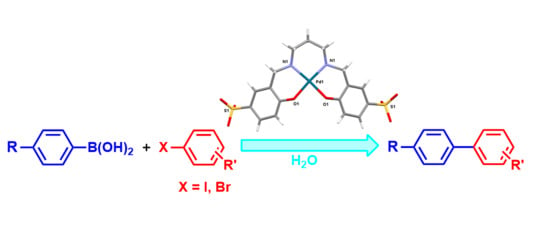
3.2. Synthesis of the Pd–Sulfosalan Complexes
3.2.1. Synthesis of Na2[Pd(PrHSS)]
In water (4 mL), 106.75 mg (0.24 mmol) of PrHSS and 73.9 mg (0.26 mmol) of (NH4)2[PdCl4] were dissolved. The pH was set to 7.5 with concentrated NaOH, and the reaction mixture was stirred at 60 °C for 10 h. Then, the solution was cooled to room temperature, and Na2[Pd(PrHSS)] was precipitated by addition of 25 mL ice-cold ethanol. The solid was filtered, washed with absolute ethanol and dried under vacuum.
Yield: 129 mg (0.22 mmol), 92%, yellow solid.
1H-NMR (D2O, 273 K, 360 MHz, δ): 1.37–1.49 (m, 1H, −CH2CH2−), 1.89 (d, J = 15.9 Hz, 1H, −CH2CH2−), 2.48 (d, J = 12.7 Hz, 2H, −CH2CH2−), 2.73 (t, J = 12.4 Hz, 2H, CH2CH2), 3.23 (d, J = 12.5 Hz, 2H, CH2−NH), 3.29 (d, J = 12.5 Hz, 2H, CH2−NH), 6.84 (d, J = 9.0 Hz, 2H, CHarom), 7.36 (s, 2H, CHarom), 7.44–7.47 (m, 2H, CHarom).
13C{1H} NMR (D2O, 90 MHz, δ/ppm): 167.27, 130.40, 128,17, 127.69, 126.47, 118.43, 52.47, 51.98, 26.67.
IR (ATR), ν/cm−1: 602.5, 707.8, 823.7, 1038.48, 1103.5, 1198.2, 1290.5, 1477.9
ESI-MS C17H18N2O8S2PdNa2 (m/z): calcd for [M − Na+]− 570.945, found 570.945.
3.2.2. Synthesis of Na2[Pd(dPhHSS)]
According to
Section 3.2.1, 140.32 mg (0.24 mmol) of dPhHSS and 73.9 mg (0.26 mmol) of (NH
4)
2[PdCl
4] yielded 149 mg (0.20 mmol), 84%, yellow solid.
1H-NMR (D2O, 298K, 360 MHz, δ): 2.95 (d, J = 13.0 Hz, 1H, CH2–NH), 3.22 (d, J = 13.0 Hz, 1H, CH2–NH), 3.80 (d, J = 13.0, 1H, CH2–NH), 4.25 (d, J = 13.0, 1H, CH2–NH), 4.29 (d, J = 4.0 Hz, 1H, CH–CH), 4.61 (d, J = 4.0 Hz 1H, CH–CH), 6.88–7.55 (m, 16H, CHarom).
13C{1H} NMR (D2O, 90 MHz, δ): 164.47, 163.66, 130.46, 128.86, 128.68, 128.01, 127.58, 126.89, 126.67, 126.55, 123.15, 121.46, 117.98, 117.69, 72.85, 70.36, 51.39, 48.12.
IR (ATR), ν/cm−1: 605.8, 708.8, 1028.7, 1106.1, 1175.4, 1300.8, 1473.1, 1592.7
ESI-MS for C28H24Na2N2O8S2Pd (m/z): calcd for [M − 2Na]2− 343.001; found 342.992.
3.2.3. Synthesis of Na2[Pd(cis-CyHSS)]
According to
Section 3.2.1, 116.77 mg (0.24 mmol) of
cis-CyHSS and 73.9 mg (0.26 mmol) of (NH
4)
2[PdCl
4] yielded 121 mg (0.19 mmol), 79%, yellow solid.
1H-NMR (D2O, 298 K, 360 MHz, δ): 1.39–2.21 (m, 8H, CH2–CH2), 3.59–3.69 (m, 4H, CH2–NH), 4.44–4.47 (m, 2H CH–CH), 6.89–6.91 (m, 2H, CHarom), 7.53–7.57 (m, 4H, CHarom).
1H-NMR (D2O, 268 K, 360 MHz, δ): 1.19–2.01 (m, 8H, CH2–CH2), 3.14 (d, J = 13.4, 1H, CH2–N), 3.41 (d, J2 = 12.9 Hz, 2H, CH–CH), 3.47 (d, J = 13.4, 1H, CH2–N), 3.89 (d, J = 13.4, 1H, CH2–N), 4.24 (d, J = 13.4, 1H, CH2–N), 6.70 (d, J = 8.4 Hz, 2H, CHarom), 7.32–7.38 (m, 4H, CHarom).
13C{1H} NMR (D2O, 90 MHz), δ: 165.52, 129.41, 127.70, 123.60, 118.70, 65.11, 51.49, 24.26, 20.61.
IR (ATR), ν/cm−1: 596.1, 634.3, 707.9, 1028.4, 1107.2, 1170.8, 1301.2, 1475.2, 1592.5
ESI-MS for C20H22Na2N2O8S2Pd (m/z): calcd for [M + Na]+ 656.954; found 656.956.
3.2.4. Synthesis of [Pd(trans-CyHSS)]
According to
Section 3.2.1, 116.77 mg (0.24 mmol) of
trans-CyHSS and 73.9 mg (0.26 mmol) of (NH
4)
2[PdCl
4] yielded 132 mg (0.21 mmol), 88%, yellow solid.
1H-NMR (D2O, 298 K, 360 MHz, δ): 1.25 (s, 4H, CH2–CH2), 1.80 (s, 2H, CH2–CH2), 2.51 (s, 2H CH2–CH2), 2.79 (s, 2H, CH2–CH2), 3.74 (d, J = 13.3 Hz, 2H, CH2–NH), 4.17 (d, J = 13.3 Hz, 2H, CH–NH), 6.87–6.90 (m, 2H, CHarom), 7.52–7.55 (m, 4H, CHarom).
1H-NMR (D2O, 268 K, 360 MHz, δ): 0.75 (s, 4H, CH2–CH2), 1.30 (s, 2H, CH2–CH2), 2.04 (s, 2H CH2–CH2), 2.30 (s, 2H, CH–CH), 3.28 (d, J = 13.3 Hz, 2H, CH2–NH), 3.68 (d, J = 13.3 Hz, 2H, CH–NH), 6.38 (d, J = 8.3 Hz, 2H, CHarom), 7.01–7.04 (m, 4H, CHarom).
13C{1H} NMR (D2O, 90 MHz, δ): 16.54, 129.40, 128.29, 127.72, 123.53, 118.74, 67.34, 50.29, 29.44, 24.14.
IR (ATR), ν/cm−1: 606.7, 708.8, 1033.3, 1105.3, 1165.3, 1298.4, 1473.3, 1590.4
ESI-MS for C20H22Na2N2O8S2Pd (m/z): calcd for [M + Na]+ 656.954; found 656.956.
3.2.5. Preparation of Pd–Salan Stock Solutions
In water (10 mL), 0.1 mmol of the appropriate salan and 28.4 mg (0.1 mmol) of (NH
4)
2[PdCl
4] were dissolved. The pH was set to 7.5 with 5 M NaOH, and the solution was stirred at 60 °C for 10 h. With time, the light brown solution turned bright yellow. Aliquots of such stock solutions of the catalysts were added to the C–C cross-coupling reaction mixtures.
1H-NMR spectra of these stock solutions are identical to those prepared by dissolution of isolated complexes (
Figures S110 and S111).