Cytospyrone and Cytospomarin: Two New Polyketides Isolated from Mangrove Endophytic Fungus, Cytospora sp. †
Abstract
:1. Introduction
2. Results and Discussion
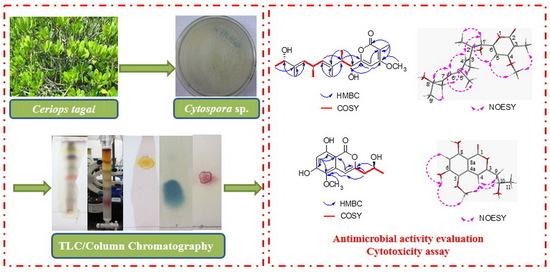
2.1. Isolation and Structure Elucidation
2.2. Bioactivities
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material and Identification
3.3. Fermentation, Extraction and Isolation
3.4. ECD Calculations
3.5. Optical Rotation (OR) Calculations
3.6. Antimicrobial Activity
3.7. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ancheeva, E.; Daletos, G.; Proksch, P. Lead compounds from mangrove-associated microorganisms. Mar. Drugs 2018, 16, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J. Bioactive natural products derived from mangrove-associated microbes. RSC Adv. 2015, 5, 841–892. [Google Scholar] [CrossRef]
- Xu, J. Biomolecules produced by mangrove-associated microbes. Curr. Med. Chem. 2011, 18, 5224–5266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, J. Chemistry and biodiversity of Rhizophora-derived endophytic fungi. In Mangrove Ecosystem Ecology and Function; IntechOpen: London, UK, 2018; Volume 8, pp. 165–186. [Google Scholar]
- Debbab, A.; Aly, A.H.; Proksch, P. Mangrove derived fungal endophytes-a chemical and biological perception. Fungal Divers. 2013, 61, 1–27. [Google Scholar] [CrossRef]
- Deng, Q.; Yang, X.B.; Lv, X.B.; Xu, J. Biomolecules produced by mangrove Ceriops tagal endophytic fungi. Chin. J. Antibiot. 2018, 43, 635–643. [Google Scholar]
- Xu, Z.Y.; Xiong, B.X.; Xu, J. Chemical investigation of secondary metabolites produced by mangrove endophytic fungus Phyllosticta capitalensis. Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef]
- Zhou, J.; Li, G.; Deng, Q.; Zheng, D.Y.; Yang, X.B.; Xu, J. Cytotoxic constituents from the mangrove endophytic Pestalotiopsis sp. induce G(0)/G(1) cell cycle arrest and apoptosis in human cancer cells. Nat. Prod. Res. 2018, 32, 2968–2972. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Wu, X.; Li, G.; Feng, Z.; Xu, J. Pestalotiopisorin B, a new isocoumarin derivative from the mangrove endophytic fungus Pestalotiopsis sp. HHL101. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Hemberger, Y.; Xu, J.; Wray, V.; Proksch, P.; Wu, J.; Bringmann, G. Pestalotiopens A and B: Stereochemically challenging flexible sesquiterpene-cyclopaldic acid Hybrids from Pestalotiopsis sp. Chem. Eur. J. 2013, 19, 15556–15564. [Google Scholar] [CrossRef]
- Deng, Q.; Li, G.; Sun, M.Y.; Yang, X.B.; Xu, J. A new antimicrobial sesquiterpene isolated from endophytic fungus Cytospora sp. from the Chinese mangrove plant Ceriops tagal. Nat. Prod. Res. 2018, 34, 1404–1408. [Google Scholar] [CrossRef]
- Bradamante, S.; Barenghi, L.; Beretta, G.; Bonfa, M.; Rollini, M.; Manzoni, M. Production of lovastatin examined by an integrated approach based on chemometrics and DOSY-NMR. Biotechnol. Bioeng. 2002, 80, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Zink, D.L.; Bills, G.F.; Pelaez, F.; Teran, A.; Collado, J.; Silverman, K.C.; Lingham, R.B.; Felock, P.; Hazuda, D.J. Discovery, structure and HIV-1 integrase inhibitory activities of integracins, novel dimeric alkyl aromatics from Cytonaema sp. Tetrahedron Lett. 2002, 43, 1617–1620. [Google Scholar] [CrossRef]
- Cai, R.L.; Wu, Y.N.; Chen, S.H.; Cui, H.; Liu, Z.M.; Li, C.Y.; She, Z.G. Peniisocoumarins A–J: Isocoumarins from Penicillium commune QQF-3, an endophytic fungus of the mangrove plant Kandelia candel. J. Nat. Prod. 2018, 81, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Savi, D.C.; Shaaban, K.A.; Mitra, P.; Ponomareva, L.V.; Thorson, J.S.; Glienke, C.; Rohr, J. Secondary metabolites produced by the citrus phytopathogen Phyllosticta citricarpa. J. Antibiot. 2019, 72, 306–310. [Google Scholar] [CrossRef]
- Xu, J. Natural Products of Mangrove-Derived Microbes; Science Press: Beijing, China, 2014; ISBN 97-7-03-041971-2. [Google Scholar]
- Xu, J.; Aly, A.H.; Wray, V.; Proksch, P. Polyketide Derivatives of endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. Tetra Lett. 2011, 52, 21–25. [Google Scholar] [CrossRef]
- Xu, J.; Kjer, J.; Sendker, J.; Wray, V.; Proksch, P. Cytosporones, coumarins, and an alkaloid from the endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. Bioorg. Med. Chem. 2009, 17, 7362–7367. [Google Scholar] [CrossRef]
- Xu, J.; Kjer, J.; Sendker, J.; Wray, V.; Proksch, P. Chromones from the endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. J. Nat. Prod. 2009, 72, 662–665. [Google Scholar] [CrossRef]
- Thongbai, B.; Surup, F.; Mohr, K.; Kuhnert, E.; Hyde, K.D.; Stadler, M. Gymnopalynes A and B, chloropropynylisocoumarin antibiotics from cultures of the basidiomycete Gymnopus sp. J. Nat. Prod. 2013, 76, 2141–2144. [Google Scholar] [CrossRef]
- Tetsuo, K.; Nigel, C.V.; Paul, D.B.; Monique, S.J.S. Dihydroisocoumarins and a tetralone from Cytospora eucalypticola. Phytochemistry 2003, 62, 779–782. [Google Scholar]
- Takano, T.; Koseki, T.; Koyama, H.; Shiono, Y. A new cytosporone derivative from the endophytic fungus Cytospora sp. Nat. Prod. Commun. 2014, 9, 973–975. [Google Scholar] [CrossRef] [Green Version]
- Brady, S.F.; Wagenaar, M.M.; Singh, M.P.; Janso, J.E.; Clardy, J. The cytosporones, new octaketide antibiotics isolated from an endophytic fungus. Org. Lett. 2000, 23, 4043–4046. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Huang, X.Y.; Li, J.; Xin, G.R.; Guo, Y.W. Absolute configurations of integracins A, B, and 15’-dehydroxy-integracin B. Chirality 2012, 24, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Xu, M.J.; Bayer, M.; Deng, Z.W.; Kubbutat, M.H.G.; Waejen, W.; Proksch, P.; Lin, W.H. Phenolic compounds and their anti-oxidative properties and protein kinase inhibition from Chinese mangrove plant Laguncularia racemose. Phytochemistry 2010, 71, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Diao, X.P.; Wang, T.; Chen, G.Y.; Lin, Q.; Yang, X.B.; Xu, J. Phylogenetic diversity and antioxidant activities of culturable fungal endophytes associated with the mangrove species Rhizophora stylosa and R. mucronata in the South China Sea. PLoS ONE 2018, 13, e0197359. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |




| MIC (mM) | |||||||
|---|---|---|---|---|---|---|---|
| Compound | E. coli GIM1.201 | MRSA GIM1.771 | P. aeruginosa GIM1.200 | C. albicans GIM2.169 | B. subtilis | C. gloeosporioides | M. oryzae |
| 1 | - | - | - | - | - | - | - |
| 2 | 0.35 | - | - | - | - | - | 1.41 |
| 3 | - | 1.94 | - | - | - | - | |
| 4 | - | - | - | - | - | - | - |
| 5 | - | - | - | - | - | - | - |
| amphotericin | - | - | - | 45.7 μM | - | 7.6 μM | 7.6 µM |
| ciprofloxacin | 0.07 | 0.14 | 0.07 | - | 0.14 | - | - |
| Cell Line | Compound | ||||
|---|---|---|---|---|---|
| 1–2 | 3a | 4a | 5a | 5-FUa | |
| HepG2(IC50) | - | 297.97 ± 17.54 | 5.98 ± 0.12 | 9.97 ± 0.06 | 43.50 ± 3.69 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, C.; Deng, Q.; Sun, M.; Xu, J. Cytospyrone and Cytospomarin: Two New Polyketides Isolated from Mangrove Endophytic Fungus, Cytospora sp. . Molecules 2020, 25, 4224. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25184224
Wei C, Deng Q, Sun M, Xu J. Cytospyrone and Cytospomarin: Two New Polyketides Isolated from Mangrove Endophytic Fungus, Cytospora sp. . Molecules. 2020; 25(18):4224. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25184224
Chicago/Turabian StyleWei, Chengwen, Qin Deng, Mengyu Sun, and Jing Xu. 2020. "Cytospyrone and Cytospomarin: Two New Polyketides Isolated from Mangrove Endophytic Fungus, Cytospora sp. " Molecules 25, no. 18: 4224. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25184224