Cytochrome P450 Can Epoxidize an Oxepin to a Reactive 2,3-Epoxyoxepin Intermediate: Potential Insights into Metabolic Ring-Opening of Benzene
Abstract
:1. Introduction
2. Results and Discussion
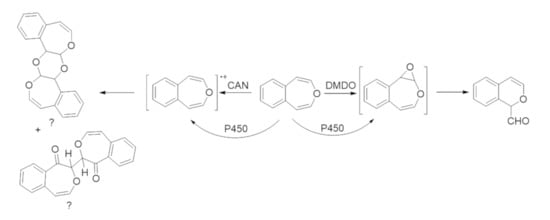
2.1. Oxidations of 4,5-Benzoxepin with DMDO and CAN
2.2. Incubations of 4,5-Benzoxepin with P450 Isoforms
2.3. Incubations of 2,7-Dimethyloxepin with P450 Isoforms
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Synthetic Procedures and Characterization
Preparation of 4,5-Benzoxepin (11) via Autooxidation
Preparation of 2,7-Dimethyloxepin (8)
Preparation of 20
Oxidation of 11 with CAN Preparation of 21
General Enzyme Incubation Procedure
References
- Vogel, E.; Günther, H. Benzene Oxide-Oxepin Valence Tautomerism. Angew. Chem. Int. Ed. Engl. 1967, 6, 385–401. [Google Scholar] [CrossRef]
- Davies, S.G.; Whitham, G.H. Benzene Oxide-Oxepin Oxidation to Muconaldehyde. J. Chem. Soc. Perkin Trans. 1977, 1, 1346–1347. [Google Scholar] [CrossRef]
- Witz, G.; Latriano, L.; Goldstein, B.D. Metabolism and Toxicity of trans, trans-Muconaldehyde, an Open-Ring Microsomal Metabolite of Benzene. Environ. Health Persp. 1989, 82, 19–22. [Google Scholar]
- Snyder, R.; Witz, G.; Goldstein, B.D. The Toxicology of Benzene. Environ. Health Persp. 1993, 100, 293–306. [Google Scholar] [CrossRef]
- Snyder, R.; Hedli, C.C. An Overview of Benzene Metabolism. Environ. Health Persp. 1996, 104, 1165–1171. [Google Scholar]
- Snyder, R. Benzene’s Toxicity: A Consolidated Short Review of Human and Animal Studies by H.A. Khan. Hum. Exp. Toxicol. 2007, 26, 687–696. [Google Scholar] [CrossRef]
- Lovern, M.R.; Turner, M.J.; Meyer, M.; Kedderis, G.L.; Bechtold, W.E.; Schlosser, P.M. Identification of Benzene Oxide as a Product of Benzene Metabolism by Mouse, Rat, and Human Liver Microsomes. Carcinogenesis 1997, 18, 1695–1700. [Google Scholar] [CrossRef] [Green Version]
- Henderson, A.P.; Barnes, M.L.; Bleasdale, C.; Cameron, R.; Clegg, W.; Heath, S.L.; Lindstrom, A.B.; Rappaport, S.M.; Waidyanatha, S.; Watson, W.P.; et al. Reactions of Benzene Oxide with Thiols including Glutathione. Chem. Res. Toxicol. 2005, 18, 265–270. [Google Scholar] [CrossRef]
- Monks, T.J.; Butterworth, M.; Lau, S.S. The Fate of Benzene Oxide. Chem. Biol. Interact. 2010, 184, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Zarth, A.T.; Murphy, S.E.; Hecht, S.S. Benzene Oxide is a Substrate for Glutathione S-Transferases. Chem. Biol. Interact. 2015, 242, 390–395. [Google Scholar] [CrossRef] [Green Version]
- Weisel, C.P. Benzene Exposure: An Overview of Monitoring Methods and Their Findings. Chem. Biol. Interact. 2010, 184, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomida, I.; Nakajima, M.Z. The Chemistry of 3,5-Cyclohexadiene-1,2-diol VI. Metabolism of the Glycols and Muconic Dialdehyde. Physiol. Chem. 1960, 318, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.D.; Witz, G.; Javid, J.; Amaruso, M.A.; Rossman, T.; Wolder, B. Muconaldehyde, a Potential Toxic Metabolite of Benzene Metabolism. Adv. Exp. Med. Biol. 1982, 136 Pt A, 331–339. [Google Scholar]
- Latriano, L.; Goldstein, B.D.; Witz, G. Formation of Muconaldehyde, an Open-Ring Metabolite of Benzene, in Mouse Liver Microsomes: An Additional Pathway for Toxic Metabolism. Proc. Natl. Acad. Sci. USA 1986, 83, 8356–8360. [Google Scholar] [CrossRef] [Green Version]
- Grotz, V.L.; Ji, S.C.; Kline, S.A.; Goldstein, B.D.; Witz, G. Metabolism of Benzene and trans, trans-Muconaldehyde in the Isolated Perfused Rat Liver. Toxicol. Lett. 1994, 70, 281–290. [Google Scholar] [CrossRef]
- Amin, D.P.; Witz, G. DNA-Protein Crosslink and DNA Strand Break Formation in HL-60 Cells Treated with trans, trans-Muconaldehyde, Hydroquinone, and Their Mixture. Int. J. Toxicol. 2001, 20, 69–80. [Google Scholar] [PubMed]
- Rivedal, E.; Leithe, E. The Benzene Metabolite trans, trans-Muconaldehyde Blocks Gap Junction Intercellular Communication by Cross-Linking Connexin 43. Toxicol. Appl. Pharmacol. 2008, 232, 463–468. [Google Scholar] [CrossRef]
- Rivedal, E.; Witz, G.; Leithe, E. Gap Unction Intercellular Communication and Benzene Toxicity. Chem. Biol. Interact. 2010, 184, 229–232. [Google Scholar] [CrossRef]
- Nakajima, T.; Wang, R.-S.; Elovaara, E.; Park, S.S.; Gelboin, H.V.; Hietanen, E.; Vainio, H. Monoclonal Antibody-Directed Characterization of Cytochrome P450 Isozymes Responsible for Toluene Metabolism in Rat Liver. Biochem. Pharmacol. 1991, 41, 395–404. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Goldstein, B.D.; Witz, G. Iron-Stimulated Ring-Opening of Benzene in a Mouse Liver Microsomal System: Mechanistic Studies and Formation of a New Metabolite. Biochem. Pharmacol. 1995, 50, 1607–1617. [Google Scholar]
- Klotz, B.; Volkamer, R.; Hurley, M.D.; Anderson, M.P.S.; Nielsen, O.J.; Barnes, I.; Imamura, T.; Wirtz, K.; Becker, K.H.; Platt, U.; et al. OH-Initiated Oxidation of Benzene. Part II. Influence of Elevated NOx Concentrations. Phys. Chem. Chem. Phys. 2002, 4, 4399–4411. [Google Scholar] [CrossRef]
- Golding, B.T.; Kennedy, G.; Watson, W.P. Simple Synthesis of Isomers of Muconaldehyde and 2-Methylmuconaldehyde. Tetrahedron Lett. 1988, 29, 5991–5994. [Google Scholar] [CrossRef]
- Golding, B.T.; Bleasdale, C.; MacGregor, J.O.; Nieschalk, J.; Pearce, K.; Watson, W.P. Chemistry of Muconaldehyde of Possible Relevance to the Toxicology of Benzene. Environ. Health Perspect. 1996, 104, 1201–1209. [Google Scholar]
- Greenberg, A.; Bock, C.W.; George, P.; Glusker, J.P. Energetics of the Metabolic Production of (E,E)-Muconaldehyde from Benzene via the Intermediates 2,3-Epoxyoxepin and (Z,Z)- and (E,Z)-Muconaldehyde: Ab Initio Molecular Orbital Calculations. Chem. Res. Toxicol. 1993, 6, 701–710. [Google Scholar] [CrossRef]
- Rosner, P.; Wolff, C.; Tochtermann, W. Syntheses of Medium and Large Rings. III. Reactions of β,β’ Hexano-Bridged Oxepins. Chem. Ber. 1982, 115, 1162–1169. [Google Scholar]
- Morgan, J.P.; Greenberg, A. Insights into the Formation and Isomerization of the Benzene Metabolite Muconaldehyde and Related Molecules: Comparison of Computational and Experimental Studies of Simple, Benzo-Annelated, and Bridged 2,3-Epoxyoxepins. J. Org. Chem. 2010, 75, 4761–4768. [Google Scholar] [CrossRef]
- Greenberg, A.; Ozari, A.; Carlin, C.M. Reactions of 2,7-Dimethyloxepin with Dimethyldioxirane and Methyl(trifluoromethyl)dioxirane: Ring Opening and Probable Observation of the Intermediate “2,3-Epoxyoxepin”. Struct. Chem. 1998, 9, 223–236. [Google Scholar] [CrossRef]
- Bleasdale, C.; Cameron, R.; Edwards, C.; Golding, B.T. Dimethyldioxirane Converts Benzene Oxide/Oxepin into (Z,Z)-Muconaldehyde and sym-Oxepin Oxide: Modeling the Metabolism of Benzene and its Photooxidation Degradation. Chem. Res. Toxicol. 1997, 10, 1314–1318. [Google Scholar] [CrossRef]
- Nauduri, D.; Greenberg, A. Direct Observation by 1H-NMR of 4,5-Benzoxepin-2,3-oxide and its Surprisingly Rapid Ring-Opening Rearrangement to 1H-2-Benzopyran-1-carboxaldehyde. Tetrahedron Lett. 2004, 45, 4789–4793. [Google Scholar] [CrossRef]
- Golding, B.T.; Barnes, M.L.; Bleasdale, C.; Henderson, A.P.; Jiang, D.; Li, X.; Mutlu, E.; Petty, H.J.; Sadeghi, M.M. Modeling the formation and reactions of benzene metabolites. Chem. Biol. Interact. 2010, 184, 196–200. [Google Scholar] [CrossRef]
- Morgan, J.P.; Greenberg, A. Curtin-Hammett Principle: Application to Benzene Oxide-Oxepin Tautomers. Struct. Chem. 2013, 24, 1945–1956. [Google Scholar] [CrossRef]
- Greenberg, A.; Bock, C.W.; George, P.; Glusker, J.P. Mechanism of Metabolic Ring-Opening of Benzene and its Relation to Mammalian PAH Metabolism. Polycycl. Aromat. Compd. 1994, 7, 123–128. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D. The Changing Face of Arene Oxide-Oxepine Chemistry. Chem. Soc. Rev. 1996, 25, 289–296. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Spartan 14, 1.1.0; Wavefunction, Inc.: Irvine, CA, USA, 2014.
- Suzuki, J.; Watanabe, T.; Suzuki, S. Formation of Mutagens by Photochemical Reaction of 2-Naphthol in Aqueous Nitrile Solution. Chem. Pharm. Bull. 1988, 36, 2204–2211. [Google Scholar] [CrossRef] [Green Version]
- Hauser, F.M.; Baghdanov, V.M. A New Procedure for Regiospecific Syntheses of Benzopyran-1-ones. J. Org. Chem. 1988, 53, 4676–4681. [Google Scholar] [CrossRef]
- Thazana, N.; Worayuthakarn, R.; Kradanrat, P.; Hohn, E.; Young, L.; Ruchirawat, S. Copper(I)-Mediated and Microwave-AssistedCaryl-Carboxylic Coupling: Synthesis of Benzopyranones and Isolamellarin Alkaloids. J. Org. Chem. 2007, 72, 9379–9382. [Google Scholar] [CrossRef]
- Cariou, M.; Carlier, R.; Simonet, J. Enamines and Enediamines: Synthesis and Anodic Oxidation. Application to the Formation of Novel Heterocycles. Bull. Soc. Chim. France 1986, 67, 81–792. [Google Scholar]
- Fukuyama, K.; Fujita, H.; Tokushima, S.; Tsukihara, T.; Katsube, Y.; Motoyama, T. Crystal and Molecular Structures of the Adduct Fluoreno[9,1-bc]pyrylium-3-olate with Methyl Cinnamate and the Dimer of Fluoreno[9,1-bc]-pyrylium-3-olate. Bull. Chem. Soc. Jpn. 1986, 59, 255–258. [Google Scholar] [CrossRef]
- Cambie, R.C.; Joblin, K.N.; Preston, A.F. Chemistry of the Podocarpaceae. XLIII. Utilization of the 8α,13- epoxylabd-14-ene and Related Compounds for the Preparation of Ambergris-Type Compounds. Aust. J. Chem. 1972, 25, 1767–1778. [Google Scholar] [CrossRef]
- Fuson, R.C.; McBurney, C.H.; Holland, W.E. 1,2-Diacylethylene Glycols. J. Am. Chem. Soc. 1939, 61, 3246–3249. [Google Scholar] [CrossRef]
- Tochtermann, W.; Rösner, P. Eine Möglichkeit zur Ringerweiterung des Cyclooctins: Synthese und Reaktionen von 3,6-Hexano-oxepin-4,5-dicarbonsäure-diethylester. Tetrahedron Lett. 1980, 21, 4905–4908. [Google Scholar] [CrossRef]
- Stok, J.E.; Chow, S.; Krenske, E.H.; Soto, C.F.; Matyas, C.; Poirier, R.A.; Williams, C.M.; De Voss, J.J. Direct Observation of an Oxepin from a Bacterial Cytochrome P450-Catalyzed Oxidation. Chem. Eur. J. 2016, 22, 4408–4412. [Google Scholar] [CrossRef] [PubMed]
- Zeigler, G.R. Mechanisms of Photochemical Reactions in Solution. LVII. Photorearrangement of 1,4-Epoxy-1,4-dihydronaphthalene to Benzo[f]oxepin. J. Am. Chem. Soc. 1969, 91, 446. [Google Scholar] [CrossRef]
- Paquette, L.A.; Barrett, J.H. 2,7-Dimethyloxepin. Org. Synth. 1969, 49, 62. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weaver-Guevara, H.M.; Fitzgerald, R.W.; Cote, N.A.; Greenberg, A. Cytochrome P450 Can Epoxidize an Oxepin to a Reactive 2,3-Epoxyoxepin Intermediate: Potential Insights into Metabolic Ring-Opening of Benzene. Molecules 2020, 25, 4542. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25194542
Weaver-Guevara HM, Fitzgerald RW, Cote NA, Greenberg A. Cytochrome P450 Can Epoxidize an Oxepin to a Reactive 2,3-Epoxyoxepin Intermediate: Potential Insights into Metabolic Ring-Opening of Benzene. Molecules. 2020; 25(19):4542. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25194542
Chicago/Turabian StyleWeaver-Guevara, Holly M., Ryan W. Fitzgerald, Noah A. Cote, and Arthur Greenberg. 2020. "Cytochrome P450 Can Epoxidize an Oxepin to a Reactive 2,3-Epoxyoxepin Intermediate: Potential Insights into Metabolic Ring-Opening of Benzene" Molecules 25, no. 19: 4542. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25194542