One-Pot Multicomponent Synthesis and Cytotoxic Evaluation of Novel 7-Substituted-5-(1H-Indol-3-yl)Tetrazolo[1,5-a] Pyrimidine-6-Carbonitrile
Abstract
:1. Introduction
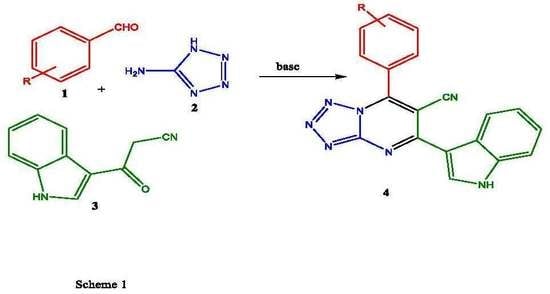
2. Results and Discussion
2.1. Chemistry
2.2. Cytotoxicity Screening
3. Structure–Activity Relationship
4. Experimental
4.1. General Methods
4.2. General Procedure for the Synthesis of 7-Substituted-5-(1H-indol-3-yl)tetrazolo[1,5-a]pyrimidine-6-carbonitrile 4a–i
4.2.1. 7-(4-Chlorophenyl)-5-(1H-indol-3-yl)tetrazolo[1,5-a]pyrimidine-6-carbonitrile 4a
4.2.2. 5-(1H-Indol-3-yl)-7-(4-nitrophenyl)tetrazolo[1,5-a]pyrimidine-6-carbonitrile 4b
4.2.3. 7-(4-Bromophenyl)-5-(1H-indol-3-yl)tetrazolo[1,5-a]pyrimidine-6-carbonitrile 4c
4.2.4. 7-(2-Hydroxyphenyl)-5-(1H-indol-3-yl)tetrazolo[1,5-a]pyrimidine-6-carbonitrile 4d
4.2.5. 5-(1H-Indol-3-yl)-7-(2-nitrophenyl)tetrazolo[1,5-a]pyrimidine-6-carbonitrile 4e
4.2.6. 5-(1H-Indol-3-yl)-7-(thiophen-2-yl)tetrazolo[1,5-a]pyrimidine-6-carbonitrile 4f
4.2.7. 7-(Furan-2-yl)-5-(1H-indol-3-yl)tetrazolo[1,5-a]pyrimidine-6-carbonitrile 4g
4.2.8. 5-(1H-Indol-3-yl)-7-(1H-pyrrol-2-yl)tetrazolo[1,5-a]pyrimidine-6-carbonitrile 4h
4.2.9. 5,7-di(1H-Indol-3-yl)tetrazolo[1,5-a]pyrimidine-6-carbonitrile 4i
4.3. Materials and Methods
4.3.1. In-Vitro Cytotoxic Activity
4.3.2. MTT Cytotoxicity Assay
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gribble, G.W. Heterocyclic Scaffolds II: Reactions and Applications of Indoles, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Yang, C.G.; Huang, H.; Jiang, B. Progress in studies of novel marine bis (indole) alkaloids. Curr. Org. Chem. 2004, 8, 1691. [Google Scholar] [CrossRef]
- Bao, B.; Sun, Q.; Yao, X.; Hong, J.; Lee, C.-O.; Sim, C.J.; Im, K.S.; Jung, J.H. Cytotoxic bisindole alkaloids from a marine sponge Spongosorites sp. J. Nat. Prod. 2005, 68, 711. [Google Scholar] [CrossRef]
- Endo, T.; Tsuda, M.; Fromont, J.; Kobayashi, J. Hyrtinadine A, a bis-indole alkaloid from a marine sponge. J. Nat. Prod. 2007, 70, 423. [Google Scholar] [CrossRef]
- Sakemi, S.; Sun, H.H. Nortopsentins A, B, and C. cytotoxic and antifungal imidazolediylbis[indoles] from the sponge spongosorites ruetzleri. J. Org. Chem. 1991, 56, 4304. [Google Scholar] [CrossRef]
- Constantinos, G.N.T.; Alexander, D. Tetrazoles via multicomponent reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar]
- Selvaraj, M.R.; Rajesh, S. Synthetic chemistry of pyrimidines and fused pyrimidines. A review. Synth. Commun. 2016, 46, 645. [Google Scholar]
- Rostom, S.A.; Ashour, H.M.; El Razik, H.A.; El-Din, N.N. Azole antimicrobial pharmacophore-based tetrazoles: Synthesis and biological evaluation as potential antimicrobial and anticonvulsant agents. Bioorg. Med. Chem. 2009, 17, 2410. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.M.H.; Khalifa, M.M.A.; AboAlzeen, R.M.; El-Torgman, A.A. Synthesis of new pyrrolo[2,3-d]pyrimidine derivatives as antibacterial and antifungal agents. Eur. J. Med. Chem. 2010, 45, 5243. [Google Scholar] [CrossRef]
- Łukowska-Chojnacka, E.; Mierzejewska, J.; Milner-Krawczyk, M.; Bondaryk, M.; Staniszewska, M. Synthesis of novel tetrazole derivatives and evaluation of their antifungal activity. Bioorg. Med. Chem. 2016, 24, 6058. [Google Scholar] [CrossRef]
- Amblard, F.; Aucagne, V.; Guenot, P.; Schinazi, R.F.; Agrofoglio, L.A. Synthesis and antiviral activity of novel acyclic nucleosides in the 5-alkynyl- and 6-alkylfuro[2,3-d]pyrimidine series. Bioorg. Med. Chem. 2005, 13, 1239. [Google Scholar] [CrossRef]
- Pandey, S.; Agrawal, P.; Srivastava, K.; RajaKumar, S.; Puri, S.K.; Verma, P.J.; Saxena, K.; Sharma, A.; Lal, J.P.; Chauhan, M.S. Synthesis and bioevaluation of novel 4-aminoquinoline-tetrazole derivatives as potent antimalarial agents. Eur. J. Med. Chem. 2013, 66, 69. [Google Scholar] [CrossRef] [PubMed]
- Frolova, L.V.; Magedov, I.V.; Romero, A.E.; Karki, M.; Otero, I.; Hayden, K.; Evdokimov, N.M.; Banuls, L.M.; Rastogi, Y.S.; Smith, W.R. Exploring natural product chemistry and biology with multicomponent reactions: Discovery of a novel tubulin-targeting scaffold derived from the rigidin family of marine alkaloids. J. Med. Chem. 2013, 56, 6886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, A.; Abdul, R.B.; Pokharel, S.; Kim, J.; Lee, E.; Athar, F.; Choi, I. Synthesis, characterization and anticancer screening of some novel piperonyl–tetrazole derivatives. Eur. J. Med. Chem. 2014, 71, 229. [Google Scholar]
- Saad, S.; Amr, N.; Abeer, M.A.; Dalia, M.A.; Ludger, A.W. Combinatorial synthesis, in silico, molecular and biochemical studies of tetrazole-derived organic selenides with increased selectivity against hepatocellular carcinoma. Eur. J. Med. Chem. 2016, 122, 55. [Google Scholar]
- Kim, T.W.; Yoo, B.W.; Lee, J.K.; Kim, J.H.; Lee, K.; Chi, Y.H.; Lee, J.Y. Synthesis and antihypertensive activity of pyrimidin-4(3H)-one derivatives as losartan analogue for new angiotensin II receptor type 1 (AT1) antagonists. Bioorg. Med. Chem. Lett. 2012, 22, 1649–1654. [Google Scholar] [CrossRef]
- Karabanovich, G.; Němeček, J.; Valášková, L.; Carazo, A.; Konečná, K.; Stolaříková, J.; Hrabálek, A.; Pavliš, O.; Pávek, P.; Vávrová, K.; et al. S-substituted 3,5-dinitrophenyl 1,3,4-oxadiazole-2-thiols and tetrazole-5-thiols as highly efficient antitubercular agents. Eur. J. Med. Chem. 2017, 126, 369. [Google Scholar] [CrossRef]
- Aponte, J.C.; Vaisberg, A.J.; Castillo, D.; Gonzalez, G.; Estevez, Y.; Arevalo, J.; Quiliano, M.; Zimic, M.; Verástegui, M.; Málaga, E.; et al. Trypanoside, anti-tuberculosis, leishmanicidal, and cytotoxic activities of tetrahydrobenzothienopyrimidines. Bioorg. Med. Chem. 2010, 18, 2880–2886. [Google Scholar] [CrossRef]
- Surmiak, E.; Neochoritis, C.G.; Musielak, B.; Twarda-Clapa, A.; Kurpiewska, K.; Dubin, G.; Camacho, C.; Holak, T.A.; Dömling, A. Rational design and synthesis of 1,5-disubstituted tetrazoles as potent inhibitors of the MDM2-p53 interaction. Eur. J. Med. Chem. 2017, 126, 384–407. [Google Scholar] [CrossRef]
- Mosaad, S.M.; Rehab, K.; Samar, S.F. Synthesis and biological evaluation of some thio containing pyrrolo [2,3-d]pyrimidine derivatives for their anti-inflammatory and anti-microbial activities. J. Med. Chem. 2010, 45, 2994. [Google Scholar]
- Yoshida, Y.; Noshiro, M.; Aoyama, Y.; Kawamoto, T.; Horiuchi, T.; Gotoh, O. Structural and evolutionary studies on sterol 14-demethylase P450 (CYP51), the most conserved P450 monooxygenase: II. Evolutionary analysis of protein and gene structures. J. Biochem. 1997, 122, 1122. [Google Scholar] [CrossRef]
- Rotstein, B.H.; Zaretsky, S.; Rai, V.; Yudin, A.K. Small heterocycles in multicomponent reactions. Chem. Rev. 2014, 114, 8323. [Google Scholar] [CrossRef]
- Peng, J.; Gao, Y.; Zhu, C.; Liu, B.; Gao, Y.; Wu, W.; Hu, M.; Jiang, H. Synthesis of polysubstituted 3-amino pyrroles via palladium-catalyzed multicomponent reaction. J. Org. Chem. 2017, 82, 3581. [Google Scholar] [CrossRef]
- Dekamin, M.G.; Eslami, M.; Maleki, A. Potassium phthalimide-N-oxyl: A novel, efficient, and simple organocatalyst for the one-pot three-component synthesis of various 2-amino-4H-chromene derivatives in water. Tetrahedron 2013, 69, 1074. [Google Scholar] [CrossRef]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958. [Google Scholar] [CrossRef]
- Ravichandiran, P.; Lai, B.; Gu, Y. Aldo-X bifunctional building blocks for the synthesis of heterocycles. Chem. Rec. 2017, 17, 142–183. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, L.; Jiang, D.; Gu, Y. Multicomponent reactions of aldo-X bifunctional reagent α-oxoketene dithioacetals and indoles or amines: Divergent synthesis of dihydrocoumarins, quinolines, furans, and pyrroles. Asian J. Org Chem. 2016, 5, 367. [Google Scholar] [CrossRef]
- Li, H.-L.; Chen, J.; Chen, D.-S.; Shi, P.; Liu, J.-Y. An efficient cascade synthesis of substituted 6,9-dihydro-1H-pyrazolo[3,4-f]quinoline- 8-carbonitriles. Heterocycl. Commun. 2018, 24, 279–283. [Google Scholar] [CrossRef]
- Wang, L.; Shi, L.X.; Liu, L.; Li, Z.X.; Xu, T.; Hao, W.J.; Li, G.; Tu, S.J.; Jiang, B. Synthesis of diastereo-enriched oxazolo[5,4b]indoles via catalyst-free multicomponent bicyclizations. J. Org. Chem. 2017, 82, 3605. [Google Scholar] [CrossRef]
- Xiong, W.N.; Yang, C.G.; Jiang, B. Synthesis of novel analogues of marine indole alkaloids: Mono(indolyl)-4-trifluoromethylpyridines and bis(indolyl)-4-trifluoromethylpyridines as potential anticancer agents. Bioorg. Med. Chem. 2001, 9, 1773–1780. [Google Scholar] [CrossRef]
- Zhu, S.L.; Ji, S.J.; Su, X.M.; Sun, C.; Liu, Y. ChemInform abstract: Facile and efficient synthesis of a new class of bis(3-indolyl)pyridine derivatives via one-pot multicomponent reactions. Tetrahedron Lett. 2008, 49, 1777–1781. [Google Scholar] [CrossRef]
- Allen, E.E.; Zhu, C.; Panek, J.S.; Schaus, S.E. Multicomponent condensation reactions via ortho-quinone methides. Org. Lett. 2017, 19, 1878–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, D.; Yadav, P.K.; Patel, O.P.S.; Parmar, N.; Maurya, R.K.; Vishwakarma, P.; Raju, K.S.R.; Taneja, I.; Wahajuddin, M.; Kar, S.; et al. Antileishmanial activity of pyrazolopyridine derivatives and their potential as an adjunct therapy with miltefosine. J. Med. Chem. 2017, 60, 1041. [Google Scholar] [CrossRef] [PubMed]
- Carbajales, C.; Jun-ichi, S.J.; Marzaro, G.; Sotelo, E.; Escalante, L.; Sánchez-Díaz Marta, A.; García-Mera, X.; Asai, A.; Coelho, A. Multicomponent assembly of the kinesin spindle protein inhibitor CPUYJ039 and analogues as antimitotic agents. ACS Comb. Sci. 2017, 19, 153. [Google Scholar] [CrossRef] [PubMed]
- Asadollah, H.; Behnam, G. An efficient regioselective three-component synthesis of tetrazoloquinazolines using g-C3N4 covalently bonded sulfamic acid. Polyhedron 2020, 175, 114217. [Google Scholar] [CrossRef]
- Siddiqui, I.R.; Shamshun, N.; Malik, A.; Hozeyfa, S. Aqua mediated multicomponent reaction under phase transfer catalysis: A novel and green approach to access fused pyrazoles. Arab. J. Chem. 2019, 12, 3254–3262. [Google Scholar] [CrossRef] [Green Version]
- Shaik, F.; Basha, T.; Nagendra, P.; Shaik, A. An efficient, multicomponent, green protocol to access 4, 7-dihydrotetrazolo [1, 5- a ] pyrimidines and 5,6,7,9-tetrahydrotetrazolo[5,1- b ]quinazolin-8(4H)-ones using PEG-400 under microwave irradiation. Synth. Commun. 2019, 49, 3181–3190. [Google Scholar] [CrossRef]
- Navaneetha, D.; Harikrishna, E. One-pot three-component synthesis of 3-aminoalkyl indoles catalyzed by molecular iodine. ChemistrySelect 2019, 4, 9722–9725. [Google Scholar] [CrossRef]
- Azees Khan, H.; Ummer, M.R.; Dharmasivam, M.; Aziz, K.R. DNA profiling and in vitro cytotoxicity studies of tetrazolo[1,5-a]pyrimidine-based copper(II) complexes. BioMetals 2019, 3, 611–626. [Google Scholar] [CrossRef]
- Gribble, G.W. Recent developments in indole ring synthesis—Methodology and applications. J. Chem. Soc. Perkin Trans. 2000, 1, 145. [Google Scholar] [CrossRef]
- Radwan, M.A.; El-Sherbiny, M. Synthesis and antitumor activity of indolylpyrimidines: Marine natural product meridianin D analogues. Bioorg. Med. Chem. 2007, 15, 1206–1211. [Google Scholar] [CrossRef]
- Radwan, M.A.; Ragab, E.A.; Sabry, N.M.; El-Shenawy, S.M. Synthesis and biological evaluation of new 3-substituted indole derivatives as potential anti-inflammatory and analgesic agents. Bioorg. Med. Chem. 2007, 15, 3832–3841. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.A.A.; Ragab, E.A.; Shaaban, M.R.; El-Nezhawy, A.O.H. Application of (2Z)-3-dimethylamino-2-(1H-indole-3-carbonyl) acrylonitrile in the synthesis of novel 3-heteroarylindoles: Condensed meridianine analogs. Arkivoc 2009, 7, 281. [Google Scholar]
- El-Nezhawy, A.O.; Eweas, A.F.; Radwan, M.A.; El-Naggar, T.B. Synthesis and molecular docking studies of novel 2-phenyl-4-substituted oxazole derivatives as potential anti-cancer agents. J. Heterocycl. Chem. 2016, 53, 271–279. [Google Scholar] [CrossRef]
- Fakhr, I.M.; Radwan, M.A.; el-Batran, S.; Abd el-Salam, O.M.; el-Shenawy, S.M. Synthesis and pharmacological evaluation of 2-substituted benzo[b]thiophenes as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2009, 44, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.A.; Shehab, M.A.; El-Shenawy, S.M. Synthesis and biological evaluation of 5-substituted benzo [b] thiophene derivatives as anti-inflammatory agents. Mon. Chem. 2009, 140, 445–450. [Google Scholar] [CrossRef]
- Reddi, M.N.K.; Park, H.; Lee, H.R.; Suh, H.; Kim, I. Efficient, solvent-free, multicomponent method for organic-base-catalyzed synthesis of b-phosphonomalonates. ACS Comb. Sci. 2015, 17, 691–697. [Google Scholar] [CrossRef]
- Hassan, A.S.; Mady, M.F.; Awad, H.M.; Hafez, T.S. Synthesis and antitumor activity of some new pyrazolo [1, 5-a] pyrimidines. Chin. Chem. Lett. 2017, 28, 388–393. [Google Scholar] [CrossRef]
- Emam, A.N.; Loutfy, S.A.; Mostafa, A.A.; Awad, H.M.; Mohamed, M.B. Cyto-toxicity, biocompatibility and cellular response of carbon dots–plasmonic based nano-hybrids for bioimaging. RSC Adv. 2017, 7, 23502–23514. [Google Scholar] [CrossRef] [Green Version]
- Flefel, E.M.; El-Sayed, W.A.; Mohamed, A.M.; El-Sofany, W.I.; Awad, H.M. Synthesis and anticancer activity of new 1-thia-4-azaspiro[4.5]decane, their derived thiazolopyrimidine and 1,3,4-thiadiazole thioglycosides. Molecules 2017, 22, 170. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |









| Entry | Base (mmol) | Solvent | Temp. (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 | - | EtOH | 80 | 10 | - |
| 2 | Et3N | EtOH | 80 | 10 | Trace |
| 3 | Et3N | CH3CN | 80 | 10 | 12 |
| 4 | Et3N | 1,4-Dioxane | 80 | 10 | 15 |
| 5 | Et3N | Toluene | 120 | 10 | - |
| 6 | Et3N | DMF | 120 | 10 | 76 |
| 7 | Piperidine 0.5 | DMF | 120 | 10 | 65 |
| 8 | DMAP 0.2 | DMF | 120 | 10 | 40 |
| 9 | Pyridine | DMF | 120 | 10 | 35 |
| 10 | KOH | CH3CN | 80 | 8 | 22 |
| 11 | NaOH | CH3CN | 80 | 8 | 27 |
| 12 | K2CO3 | CH3CN | 80 | 8 | Trace |
| Compound Code | IC50 (µM) ± SD | ||||
|---|---|---|---|---|---|
| HCT-116 | MCF-7 | MDA-MB-231 | A549 | RPE-1 | |
| 4a | 20.4 ± 2.1 | 32.5 ± 2.4 | 21.1 ± 2.1 | 20.9 ± 2.1 | 61.5 ± 2.5 |
| 4b | 14.1 ± 1.5 | 28.1 ± 2.1 | 22.5 ± 2.9 | 22.4 ± 1.8 | 56.3 ± 3.1 |
| 4c | 14.2 ± 1.7 | 22.0 ± 1.8 | 19.4 ± 3.1 | 24.7 ± 2.3 | 42.4 ± 2.8 |
| 4d | 23.1 ± 2.2 | 25.7 ± 2.1 | 16.2 ± 2.3 | 22.7 ± 2.1 | 31.8 ± 3.1 |
| 4e | 26.6 ± 2.1 | 17.6 ± 1.5 | 19.7 ± 2.8 | 25.6 ± 1.9 | 50.9 ± 2.9 |
| 4f | 31.9 ± 2.6 | 23.9 ± 1.9 | 30.6 ± 2.2 | 25.9 ± 2.1 | 62.4 ± 2.8 |
| 4g | 28.8 ± 1.9 | 26.3 ± 2.5 | 31.1 ± 2.9 | 26.0 ± 2.3 | 60.4 ± 3.4 |
| 4h | 14.6 ± 1.5 | 26.0 ± 2.1 | 26.3 ± 1.8 | 27.5 ± 2.5 | 67.5 ± 3.9 |
| 4i | 19.9± 2.1 | 24.8 ± 2.4 | 27.4 ± 2.3 | 26.6 ± 2.1 | 77.2 ± 3.8 |
| Doxorubicin | 17.2 ± 1.5 | 17.5 ± 2.1 | - | 27.0 ± 2.4 | 66.1 ± 3.5 |
| 5-Fluorouracil | - | - | 3.8 ± 0.2 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radwan, M.A.A.; Alminderej, F.M.; Awad, H.M. One-Pot Multicomponent Synthesis and Cytotoxic Evaluation of Novel 7-Substituted-5-(1H-Indol-3-yl)Tetrazolo[1,5-a] Pyrimidine-6-Carbonitrile. Molecules 2020, 25, 255. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25020255
Radwan MAA, Alminderej FM, Awad HM. One-Pot Multicomponent Synthesis and Cytotoxic Evaluation of Novel 7-Substituted-5-(1H-Indol-3-yl)Tetrazolo[1,5-a] Pyrimidine-6-Carbonitrile. Molecules. 2020; 25(2):255. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25020255
Chicago/Turabian StyleRadwan, Mohamed A. A., Fahad M. Alminderej, and Hanem M. Awad. 2020. "One-Pot Multicomponent Synthesis and Cytotoxic Evaluation of Novel 7-Substituted-5-(1H-Indol-3-yl)Tetrazolo[1,5-a] Pyrimidine-6-Carbonitrile" Molecules 25, no. 2: 255. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25020255