Synthesis of a New Class of Spirooxindole–Benzo[b]Thiophene-Based Molecules as Acetylcholinesterase Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Analogs IIa–n
2.2. In Vitro Biological Activity
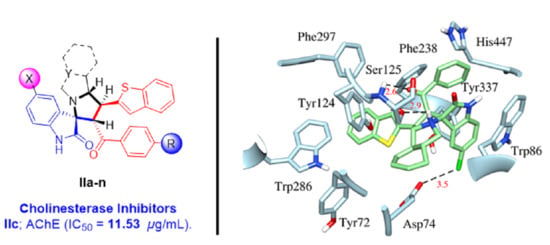
2.3. Molecular Docking Study
3. Materials and Methods
3.1. General Experimental Information
3.2. General Procedure for the Synthesis of Chalcones 2a–e
3.3. General Procedure for the Synthesis of Oxindole-Based Spiro-Heterocycles IIa–n
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schelterns, P.; Feldman, H. Treatment of Alzheimer’s disease; current status and new perspectives. Lancet Neurol. 2003, 2, 539. [Google Scholar]
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, M. Alzheimer’s Disease International; King’s College: London, UK, 2015. [Google Scholar]
- Wang, Z.; Wang, Y.; Li, W.; Mao, F.; Sun, Y.; Huang, L.; Li, X. Design, synthesis, and evaluation of multitarget-directed selenium-containing clioquinol derivatives for the treatment of Alzheimer’s disease. ACS Chem. Neurosci. 2014, 5, 952. [Google Scholar] [CrossRef]
- Savelieff, M.G.; Lee, S.; Liu, Y.; Lim, M.H. Untangling amyloid-β, tau, and metals in Alzheimer’s disease. ACS Chem. Biol. 2013, 8, 856. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, S.R.; Kim, S.U.; Lee, H.J. Alzheimer’s disease and stem cell therapy. Exp. Neurol. 2014, 23, 45. [Google Scholar] [CrossRef]
- Giacobini, E. Selective inhibitors of butyrylcholinesterase: A valid alternative for therapy of Alzheimer’s disease? Drugs Aging 2001, 18, 891. [Google Scholar] [CrossRef] [PubMed]
- Kia, Y.; Osman, H.; Kumar, R.S.; Murugaiyah, V.; Basiri, A.; Perumal, S.; Razak, I.A. A facile chemo-, regio-and stereoselective synthesis and cholinesterase inhibitory activity of spirooxindole–pyrrolizine–piperidine hybrids. Bioorganic Med. Chem. Lett. 2013, 23, 2979. [Google Scholar] [CrossRef] [PubMed]
- Kia, Y.; Osman, H.; Kumar, R.S.; Basiri, A.; Murugaiyah, V. Synthesis and discovery of highly functionalized mono- and bis-spiro-pyrrolidines as potent cholinesterase enzyme inhibitors. Bioorganic Med. Chem. Lett. 2014, 24, 1815. [Google Scholar] [CrossRef] [PubMed]
- Kia, Y.; Osman, H.; Kumar, R.S.; Murugaiyah, V.; Basiri, A.; Perumal, S.; Wahab, H.A.; Bing, C.S. Synthesis and discovery of novel piperidone-grafted mono- and bis-spirooxindole- hexahydropyrrolizines as potent cholinesterase inhibitors. Bioorganic Med. Chem. 2013, 21, 1696. [Google Scholar] [CrossRef] [PubMed]
- Kia, Y.; Osman, H.; Kumar, R.S.; Basiri, A.; Murugaiyah, V. Ionic liquid mediated synthesis of mono- and bis-spirooxindole-hexahydropyrrolidines as cholinesterase inhibitors and their molecular docking studies. Bioorg. Med. Chem. 2014, 22, 1318. [Google Scholar] [CrossRef]
- Arumugam, N.; Almansour, A.I.; Kumar, R.S.; Kotresha, D.; Saiswaroop, R.; Venketesh, S. Dispiropyrrolidinyl-piperidone embedded indeno [1,2-b] quinoxaline heterocyclic hybrids: Synthesis, cholinesterase inhibitory activity and their molecular docking simulation. Bioorg. Med. Chem. 2019, 27, 2621. [Google Scholar] [CrossRef]
- Barakat, A.; Soliman, S.M.; Alshahrani, S.; Islam, M.S.; Ali, M.; Al-Majid, A.M.; Yousuf, S. Synthesis, X-ray Single Crystal, Conformational Analysis and Cholinesterase Inhibitory Activity of a New Spiropyrrolidine Scaffold Tethered Benzo [b] Thiophene Analogue. Crystals 2020, 10, 120. [Google Scholar] [CrossRef] [Green Version]
- Goyal, D.; Kaur, A.; Goyal, B. Benzofuran and Indole: Promising Scaffolds for Drug Development in Alzheimer’s Disease. ChemMedChem 2018, 13, 1275. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Ali, M.A.; Osman, H.; Ismail, R.; Choon, T.S.; Yoon, Y.K.; Wei, A.C.; Pandian, S.; Manogaran, E. Synthesis and discovery of novel hexacyclic cage compounds as inhibitors of acetylcholinesterase. Bioorganic Med. Chem. Lett. 2011, 21, 3997. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, G.; El Ashry, E.S.H.; Said, M.M.; El Tamany, E.S.H.; Aziz, Y.M.A.; Al-Dhfyan, A.; Al-Majid, A.M.; Barakat, A. Regio-and stereoselective synthesis of new spirooxindoles via 1,3-dipolar cycloaddition reaction: Anticancer and molecular docking studies. J. Photochem. Photobiol. B Biol. 2018, 180, 98. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, G.; Said, M.M.; El Ashry, E.S.H.; El Tamany, E.S.H.; Al-Dhfyan, A.; Aziz, Y.M.A.; Barakat, A. Synthesis of new spirooxindole-pyrrolothiazole derivatives: Anti-cancer activity and molecular docking. Biorg. Med. Chem. 2017, 25, 1514. [Google Scholar] [CrossRef]
- Lotfy, G.; Aziz, Y.M.A.; Said, M.M.; El Ashry, E.S.H.; El Tamany, E.S.H.; Barakat, A.; Ghabbour, H.A.; Yousuf, S.; Ul-Haq, Z.; Choudhary, M.I. Synthesis of oxindole analogues, biological activity, and in silico studies. ChemistrySelect 2019, 4, 10510. [Google Scholar] [CrossRef]
- Keri, R.S.; Chand, K.; Budagumpi, S.; Somappa, S.B.; Patil, S.A.; Nagaraja, B.M. An overview of benzo [b] thiophene-based medicinal chemistry. Eur. J. Med. Chem. 2017, 138, 1002–1033. [Google Scholar] [CrossRef]
- Anbar, H.S.; El-Gamal, R.; Ullah, S.; Zaraei, S.O.; al-Rashida, M.; Zaib, S.; Pelletier, J.; Sevigny, J.; Iqbal, J.; El-Gamal, M.I. Evaluation of sulfonate and sulfamate derivatives possessing benzofuran or benzothiophene nucleus as inhibitors of nucleotide pyrophosphatases/phosphodiesterases and anticancer agents. Bioorganic Chem. 2020, 104, 104305. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zhang, J.; Ullah, S.; Kang, N.; Zhao, Y.; Zhou, H. Benzothiophene-2-carboxamide derivatives as SENPs inhibitors with selectivity within SENPs family. Eur. J. Med. Chem. 2020, 204, 112553. [Google Scholar] [CrossRef]
- Pieroni, M.; Azzali, E.; Basilico, N.; Parapini, S.; Zolkiewski, M.; Beato, C.; Annunziato, G.; Bruno, A.; Vacondio, F.; Costantino, G. Accepting the Invitation to Open Innovation in Malaria Drug Discovery: Synthesis, Biological Evaluation, and Investigation on the Structure–Activity Relationships of Benzo[b]thiophene-2-carboxamides as Antimalarial Agents. J. Med. Chem. 2017, 60, 1959–1970. [Google Scholar] [CrossRef]
- Qin, Z.; Kastrati, I.; Chandrasena, R.E.P.; Liu, H.; Yao, P.; Petukhov, P.A.; Bolton, J.L.; Thatcher, G.R.J. Benzothiophene Selective Estrogen Receptor Modulators with Modulated Oxidative Activity and Receptor Affinity. J. Med. Chem. 2007, 50, 2682–2692. [Google Scholar] [CrossRef] [PubMed]
- Konstantinović, J.; Videnović, M.; Srbljanović, J.; Djurković-Djaković, O.; Bogojević, K.; Sciotti, R.; Šolaja, B. Antimalarials with Benzothiophene Moieties as Aminoquinoline Partners. Molecules 2017, 22, 343. [Google Scholar] [CrossRef] [Green Version]
- El-Miligy, M.M.M.; Hazzaa, A.A.; El-Messmary, H.; Nassra, R.A.; El-Hawash, S.A.M. New hybrid molecules combining benzothiophene or benzofuran with rhodanine as dual COX-1/2 and 5-LOX inhibitors: Synthesis, biological evaluation and docking study. Bioorganic Chem. 2017, 72, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dong, G.; He, S.; Xu, T.; Wang, X.; Liu, N.; Zhang, W.; Miao, C.; Sheng, C. Identification of benzothiophene amides as potent inhibitors of human nicotinamide phosphoribosyltransferase. Bioorganic Med. Chem. Lett. 2016, 26, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Aleksić, M.; Bertoša, B.; Nhili, R.; Depauw, S.; Martin-Kleiner, I.; David-Cordonnier, M.-H.; Tomić, S.; Kralj, M.; Karminski-Zamola, G. Anilides and quinolones with nitrogen-bearing substituents from benzothiophene and thienothiophene series: Synthesis, photochemical synthesis, cytostatic evaluation, 3D-derived QSAR analysis and DNA-binding properties. Eur. J. Med. Chem. 2014, 71, 267–281. [Google Scholar] [CrossRef]
- Altowyan, M.S.; Barakat, A.; Al-Majid, A.M.; Al-Ghulikah, H.A. Spiroindolone Analogues as Potential Hypoglycemic with Dual Inhibitory Activity on α-Amylase and α-Glucosidase. Molecules 2019, 24, 2342. [Google Scholar] [CrossRef] [Green Version]
- Altowyan, M.S.; Barakat, A.; Al-Majid, A.M.; Al-Ghulikah, H.A. New Spiroindolone Bearing Benzofuran Moiety as High Effective and Selective for COX-1 Inhibitor with TNF-α, and IL-6 inhibitory activity. Saudi J. Biol. Sci. 2020, 27, 1208. [Google Scholar] [CrossRef]
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.M.; El-Senduny, F.F.; Badria, F.A.; Elshaier, Y.A.M.; Ghabbour, H.A. Substituted spirooxindole derivatives as potent anticancer agents through inhibition of phosphodiesterase 1. RSC Adv. 2018, 8, 14335. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Ghawas, H.M.; El-Senduny, F.F.; Al-Majid, A.M.; Elshaier, Y.A.; Badria, F.A.; Barakat, A. Synthesis of new thiazolo-pyrrolidine–(spirooxindole) tethered to 3-acylindole as anticancer agents. Bioorganic Chem. 2019, 82, 423. [Google Scholar] [CrossRef]
- Al-Majid, A.M.; Soliman, S.M.; Haukka, M.; Ali, M.; Islam, M.S.; Shaik, M.R.; Barakat, A. Design, Construction, and Characterization of a New Regioisomer and Diastereomer Material Based on the Spirooxindole Scaffold Incorporating a Sulphone Function. Symmetry 2020, 12, 1337. [Google Scholar] [CrossRef]
- Zhou, L.M.; Qu, R.Y.; Yang, G.F. An overview of spirooxindole as a promising scaffold for novel drug discovery. Expert Opin. Drug Discov. 2020, 15, 603–625. [Google Scholar] [CrossRef] [PubMed]
- Almansour, A.I.; Arumugam, N.; Kumar, R.S.; Kotresha, D.; Manohar, T.S.; Venketesh, S. Design, synthesis and cholinesterase inhibitory activity of novel spiropyrrolidine tethered imidazole heterocyclic hybrids. Bioorganic Med. Chem. Lett. 2020, 30, 126789. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Althomili, D.M.Q.; Altaf, M.; Basiri, A.; Manohar, T.S.; Venketesh, S. Ionic liquid-enabled synthesis, cholinesterase inhibitory activity, and molecular docking study of highly functionalized tetrasubstituted pyrrolidines. Bioorganic Chem. 2018, 77, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Lilienfeld, S. Galantamine—A novel cholinergic drug with a unique dual mode of action for the treatment of patients with Alzheimer’s disease. CNS Drug Rev. 2002, 8, 159–176. [Google Scholar] [CrossRef]
- Shang, Y.; Jie, X.; Zhou, J.; Hu, P.; Huang, S.; Su, W. Pd-Catalyzed C–H Olefination of (Hetero) Arenes by Using Saturated Ketones as an Olefin Source. Angew. Chem. Int. 2013, 52, 1299. [Google Scholar] [CrossRef]
- Wu, C.; Yue, G.; Nielsen, C.D.T.; Xu, K.; Hirao, H.; Zhou, J. Asymmetric conjugate addition of organoboron reagents to common enones using copper catalysts. J. Am. Chem. Soc. 2016, 138, 742. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds IIa–n are available from the authors. |






| # | Chemical Structures | AChE Inhibition IC50 (µM/L) |
|---|---|---|
| 1 |  IIa | 85,560 |
| 2 |  IIb | 88,410 |
| 3 |  IIc | 20,840 |
| 4 |  IId | 37,670 |
| 5 |  IIe | 50,590 |
| 6 |  IIf | 34,020 |
| 7 |  IIg | 23,040 |
| 8 |  IIh | 121,690 |
| 9 |  IIi | 72,380 |
| 10 |  IIj | 75,980 |
| 11 |  IIk | 41,530 |
| 12 |  IIl | 29,760 |
| 13 |  IIm | 41,450 |
| 14 |  IIn | 36,830 |
| STD |  Galantamine | 2090 [7,8,9,10,11] 3400 [34] 0.35 [35] |
| S.No. | Compounds Name | Scores | No. of Hydrogen Bonds |
|---|---|---|---|
| 1 | IIc | −6.54 | 3 |
| 2 | IIf | −6.01 | 3 |
| 3 | IIg | −6.23 | 3 |
| 4 | IIl | −6.19 | 4 |
| 5 | Galantamine | −9.28 | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, A.; Alshahrani, S.; Al-Majid, A.M.; Ali, M.; Altowyan, M.S.; Islam, M.S.; Alamary, A.S.; Ashraf, S.; Ul-Haq, Z. Synthesis of a New Class of Spirooxindole–Benzo[b]Thiophene-Based Molecules as Acetylcholinesterase Inhibitors. Molecules 2020, 25, 4671. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25204671
Barakat A, Alshahrani S, Al-Majid AM, Ali M, Altowyan MS, Islam MS, Alamary AS, Ashraf S, Ul-Haq Z. Synthesis of a New Class of Spirooxindole–Benzo[b]Thiophene-Based Molecules as Acetylcholinesterase Inhibitors. Molecules. 2020; 25(20):4671. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25204671
Chicago/Turabian StyleBarakat, Assem, Saeed Alshahrani, Abdullah Mohammed Al-Majid, M. Ali, Mezna Saleh Altowyan, Mohammad Shahidul Islam, Abdullah Saleh Alamary, Sajda Ashraf, and Zaheer Ul-Haq. 2020. "Synthesis of a New Class of Spirooxindole–Benzo[b]Thiophene-Based Molecules as Acetylcholinesterase Inhibitors" Molecules 25, no. 20: 4671. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25204671