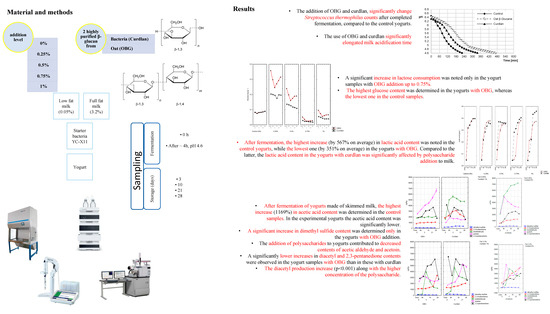
2.1. Effect of the Addition Level of Glucan Preparations on Changes in the Counts of Starter Cultures
The mean
Streptococcus thermophilus (ST) count in the control yogurts produced from skimmed milk and full-fat milk reached 8.73 log cfu/g (
Figure 1;
Table S1). The addition of OBG and curdlan led to, respectively, significantly higher (ca. 0.5 log cfu/g) and lower (ca. 0.4 log cfu/g) ST counts after completed fermentation, compared to the control yogurts. In turn, no significant differences in ST numbers were noted between experimental and control yogurts in the study with a structurally-similar polysaccharide-pullulan [
18] because of carbon sources’ presence in milk that was easier available to bacteria than pullulan.
After 28 days of storage, regardless of fat content in the raw material, significant changes were noted in the ST population in all yogurts analyzed. A significant (
p < 0.001) increase in the ST count was determined only in the yogurts with a 0.25% addition of OBG and curdlan, reaching 50% (~3 log cfu/g) and 7% (~0.6 log cfu/g) in these made of skimmed milk as well as 17% (~1.3 log cfu/g) and 5% (~3 log cfu/g) in these made of full-fat milk, respectively. These observations are consistent with findings reported by Kilic et al. [
5], who showed that a 0.25% addition of a PROMOAT preparation (with 29% content of β-glucan) increased ST population. When used in higher than 0.25% concentrations, both preparations decreased ST count, which is in line with Lazaridou et al. [
9], and Zhao et al. [
10]. In contrast to our study, the use of a 2% addition of pullulan [
18] or inulin [
19] caused no significant change in ST count compared to the control yogurt, which indicates a significant effect of preparation structure on ST metabolism.
The mean count of
Lactobacillus bulgaricus (LB) in yogurts was lower than that of ST and reached 5.19 log cfu/g in the yogurts made of skimmed milk and 4.03 log cfu/g in these made of full-fat milk (
Figure 1). These values are ca. 30% lower than data presented by Kycia et al. [
18], which is due to the use of starter cultures with different ST to LB ratios and at different addition levels. The results of the multi-way analysis of variance showed that LB count determined after 28 days of storage was significantly affected by polysaccharide type and addition level (
p < 0.01) (
Table S7), which is consistent with the findings presented by Lazaridou et al. [
9]. After 28-day storage of yogurts made of full-fat milk, the population number of LB increased by 7.30% in the samples with OBG addition and by 2.25% in these with curdlan addition. In turn, the LB count decreased slightly (
p > 0.05) in the yogurts made of skimmed milk, probably because of the smaller availability of carbon and nitrogen sources to the bacteria, compared to the full-fat milk. These results are in line with data reported by Kycia et al. [
18] for pullulan and with these presented by Kilic et al. [
14] for OBG, whose addition to milk reduced
L. plantarum count. An insignificant increase in the LB population was also demonstrated in yogurts with inulin addition [
16]. Vasiljevic et al. [
16] also concluded that OBG addition to yogurts produced from skimmed milk caused no significant LB count changes than the control yogurts, which agrees with the results of our study. In turn, Allgeyer et al. [
4] demonstrated ca. 2 log cfu/g lower viability of a probiotic
Lactobacillus acidophilus LA-5 strain after 30-day storage of yogurt drinks with maize fiber, polydextrose, and inulin. Unlike plant-based beverages, milk is a good source of carbon and nitrogen for starter cultures used to produce fermented milk beverages. According to some authors like Kilik et al. [
5], lactic acid bacteria (LAB) count increases in yogurts with polysaccharides because they synthesize enzymes enabling the degradation of β-glucans to easily-available carbon sources. However, lactose or glucose represents a carbon source more easily available to bacteria than the polysaccharide added.
2.2. Effect of β-Glucan Addition on Milk Acidification Dynamics
The pH value of control milk, milk with OBG, and milk with curdlan measured immediately after starter culture addition reached 6.46, 6.48, and 6.44, respectively, and was typical of the pH value of fresh milk after pasteurization (
Figure 2).
The use of OBG and curdlan in the concentration of 0.25% significantly elongated milk acidification time, i.e., the time needed for milk acidification to pH = 4.6 ± 0.01 (compared to the control yogurt), to 256 min and 112 min (
Figure 2A). These results are in line with the study by Kilic et al. [
14], in which the use of BG preparations in the concentrations from 0.25% to 1% slowed down yogurt acidification dynamics. Similar observations were made by Kristo et al. [
13], who demonstrated that the time needed for milk acidification to pH 4.6 was significantly longer upon using exopolysaccharide-producing strains than traditional starter cultures.
Opposite results were achieved by Lazaridou et al. [
9], who used 1.4% OBG addition and did not observe any significant changes in milk acidification dynamics. It needs to be emphasized that OBG concentrations used in the present study were lower than those used by Lazaridou et al. [
9]. Besides, in the present study, the milk acidification time shortened significantly, along with the OBG content increase in the sample (
Figure 2B–D). Presumably, increasing its content to ca. 1.4% would cause similar results as those reported by Lazaridou et al. [
9]. Our study’s results confirm that the metabolic activity of
Streptococcus thermophilus can be significantly affected by a gel’s rheological properties. Probably, the gel’s high viscosity impaired the diffusion of essential growth substrates and the removal of bacterial metabolites.
2.3. Effect of β-Glucan Addition on Sugar Metabolism
The changes in numbers of starter cultures over yogurt fermentation and storage periods are determined by the content and availability of easily-available carbon sources, i.e., carbohydrates. The mean lactose content in all experimental samples reached 48.70 g/L (
Figure 3A,
Tables S2 and S3).
The results are similar to those reported by Leite et al. [
20], but lower than those presented by Delgado-Fernández et al. [
21] for samples of yogurt made of cow’s milk with the addition of oligosaccharides (53–54 g/L). A lower mean lactose content was probably attributable to the milk chemical composition’s seasonal variations and a different normalization of the milk. Among all yogurt samples tested, the greatest (
p < 0.05) decrease in lactose content was determined in the samples between day 0 and day 10 of storage. Since day 10 of storage, changes in lactose content were small and statistically insignificant. Lactose consumption by LAB was higher by ca. 5% in the experimental yogurts with 0.5% and 0.75% OBG, compared to the control yogurt and yogurt with curdlan (
Table S6). In turn, in the samples with 1% addition of the polysaccharides, lactose consumption by LAB was comparable with that noted in the control samples (
p > 0.05). Unlike curdlan, OBG probably stimulates bacteria’s metabolic activity; hence lower lactose contents were determined in the experimental yogurts.
Our study results are consistent with those reported by Pereira da Costa et al. [
12], who noted a significant decrease in lactose content in yogurt samples with a 10% addition of cupuassu pulp. The above authors and also Zalán et al. [
22] demonstrated that the use of inulin in the production of yogurt does not significantly increase lactose metabolism, which could be due to use of the prebiotic as a carbon source by starter lactic acid bacteria (SLAB). Nevertheless, given that milk also contains glucose, the polysaccharide utilization as the first carbon source of choice seems disputable. The glucose is consumed by SLAB in metabolic processes. Our study showed its content significantly (
p < 0.01) depended on storage time and product type. Regardless of sampling time, the highest glucose content was determined in the yogurts with OBG and the lowest in the control products (
Figure 3B). In the yogurts with OBG, glucose content decreased significantly until day 3 of storage and then increased slowly. This dependency was, probably, due to an insignificantly higher LAB count in the yogurts with OBG at the early stage of storage (
Figure 1). However, the significant increase observed since day 3 of storage was most likely because the bacteria found another easily available carbon source. Compared to the yogurt with OBG, a successive, significant decrease in glucose content was noted in the yogurt with curdlan. In turn, in the control yogurts, statistically insignificant changes in its content were observed throughout the storage period. These results agree with the study by Pereira da Costa et al. [
12]. Changes in glucose content in yogurts during their storage are strongly associated with lactose metabolism. Glucose synthesized in the Embden-Meyerhoff-Parnas pathway is hydrolyzed by bacteria firstly to pyruvate and then to lactate. Therefore, changes in its content in a product may be one indicator of polysaccharide’s effect on bacteria’s metabolic activity.
2.4. Effect of β-Glucan Addition on Changes in the Contents of Organic Acids in Yogurt
Specific metabolic pathways of bacteria lead to the conversion of sugars into organic acids and volatile compounds. Depending on the strain and species composition of starter cultures, lactose fermentation may proceed via the glycolytic pathway, during which only lactic acid is produced, or via the phosphate-pentose pathway ending in the synthesis of not only lactic acid but also acetic acid [
23]. Lactic acid turned out to be the major acid among all organic acids determined (
Figure 4,
Tables S4 and S5).
The lactic acid content in the samples before the acidification process depends on the microbial quality of raw milk used to produce. In the present study, its content of lactic acid in milk before inoculation with the starter culture ranged from 0.045–0.053 g/L (
Figure 4A). A significant (
p < 0.001) increase in lactic acid content was determined in yogurt samples during the acidification process, due to the proliferation of starter cultures and lactose metabolic conversion by starter cultures to glucose and then, i.e., to lactic acid. The amount of organic acids synthesized during fermentation depends not only on the starter culture type but may be either increased or decreased by the functional additives used [
24], including OBG or curdlan.
This was also confirmed in our study, where the highest (0.271 g/L; 567% on average) increase in lactic acid content during fermentation was noted in the control yogurts and the lowest one (0.165 g/L; 351% on average) in the yogurts with OBG. Our results are similar to the results reported by Kilic et al. [
14], who showed the 0.25–1% BG addition to milk suppressed synthesis of lactic acid by starter cultures. Compared to the yogurt with OBG, the lactic acid content in the yogurts with curdlan was significantly dependent on polysaccharide addition level. The highest (0.370 g/L) lactic acid content was determined in the sample with 0.5% addition and the lowest one (0.117 g/L) in that with a 1% addition of curdlan. Worthy of attention is that over the ripening process, the increase in lactic acid content was significantly higher in the yogurt samples made of full-fat milk with OBG (0.200 g/L; 395%) than in the yogurts made of skimmed milk (0.165 g/L; 351%). Presumably, the coupling of fat globule elements released during yogurt production with OBG enhanced bacterial cultures’ metabolic activity, consistent with the study by Moe et al. [
25]. An increased mean lactic acid content, reaching 0.398, 0.371, and 0.394 g/L in the control yogurts and these with OBG and curdlan, respectively, and significantly (
p < 0.01) influenced by polysaccharide preparation type and addition level was also observed after 28-day storage. Interestingly, in the yogurts made of skimmed milk with curdlan addition, its content increased along with the polysaccharide inclusion level. The results obtained over the fermentation period and after 28-day storage point to a significant effect of the polysaccharide itself (its structure) and the concentration of a polysaccharide preparation on the course of glycolytic transformations mediated by SLAB. The above results are in line with works by Özer et al. [
6] and Li et al. [
26], who demonstrated the use of such polysaccharides as lactulose, inulin, iso-maltooligosaccharides, and xylooligosaccharides led to a significant (ca. 2 g/kg) increase in the lactic acid content of the experimental yogurts. As in the case of lactic acid, the content of acetic acid was also significantly affected by polysaccharide addition level, storage time, and fat content in the raw material used to produce yogurts. After completed fermentation of yogurts made of skimmed milk, the highest increase in acetic acid content (1169%; 3699 µg/kg) was determined in the control samples (
Figure 4B); while in the yogurts with OBG and curdlan this increase was significantly (
p < 0.01) lower and reached 100% (244 µg/kg) and 250% (1622 µg/kg) on average, respectively. In addition, acetic acid content was higher in the yogurts made of full-fat than skimmed milk, which contradicts the results reported by Kaminarides et al. [
27]. Compared to the fermented yogurt samples, in the same samples stored for 28 days, the smallest (156%; 1462 µg/kg) increased in acetic acid content was determined in the control samples, whereas the largest one (1380%; 3059 µg/kg) in the yogurt with OBG. The increased content of acetic acid in yogurts was due to various metabolic transformations of LAB involved in, i.a., fermentation of lactose and degradation of citrates or amino acids [
24]. The amount of synthesized acid was also significantly (
p < 0.01) dependent on the polysaccharide addition level. The polysaccharide addition of up to 0.75% led to an increase, whereas its higher addition at 1% caused a decrease in acetic acid content in the yogurts, which is partly in line with results obtained by Kilic et al. [
5], who demonstrated that acetic acid content was proportional to the inclusion level of an OBG preparation (PROMAT). However, in contrast to our study, the authors cited above used a preparation in which OBG accounted for only 30%. The higher acetic acid growth dynamics in the yogurt samples with OBG than curdlan agrees with other authors’ observations. The use of only apparently similar preparations of BG isolated from cereals, i.e., oats or barley, increased acetic acid content in the yogurt samples with barley β-glucan compared to these with oat β-glucan [
24]. In turn, the use of iso-maltooligosaccharides and xylooligosaccharides caused no significant changes in its content in yogurt samples [
26], whereas results of studies with inulin were inconclusive [
6,
26], probably due to various inulin preparations and starter cultures used.
2.5. Effect of β-Glucan Addition on the Changes in Contents of Volatile Compounds
Contents of volatile compounds, i.e., dimethyl sulfide, acetaldehyde, diacetyl, acetoin, and 2,3-pentanedione, in yogurts, are presented in
Figure 5. Together with organic acids and sugars, volatiles of yogurts determine their sensory quality. Dimethyl sulfide content in the yogurts made of skimmed and full-fat milk with the addition of polysaccharides was at 127 µg/kg and 116 µg/kg, and accounted for ca. 1.5% of total volatile compounds determined (
Figure 5).
After completed fermentation, a significant increase in dimethyl sulfide content was only observed in the yogurts with OBG. In the samples with curdlan, its content decreased depending on the polysaccharide addition level (
Figure 5,
Table S7). These results are similar to the findings presented by Ott et al. [
28] and significantly lower than those from the study by Martin et al. [
29], where dimethyl sulfide content in yogurts was as high as 10,000 µg/kg. This difference was attributable to a different yogurt production technique and analytical method used for dimethyl sulfide determination.
As in the fermentation case, a significant (
p < 0.01) increase was noted in dimethyl sulfide content during storage of yogurts with OBG addition of up to 0.75%, with the increase being proportional to OBG addition level. Besides, a more significant increase in dimethyl sulfide content was determined in the yogurts made of skimmed than full-fat milk. In turn, in most yogurts with curdlan, dimethyl sulfide’s content decreased significantly (~50%), proportionally to its addition level. These results are in line with those reported by Moineau-Jean et al. [
30], who also demonstrated that dimethyl sulfide content decreased by ca. 50% over a 28-day storage period.
The changes observed in dimethyl sulfide content over the storage period and its various contents in the yogurts examined are due to the various effects of the β-glucan preparations on the metabolic activity (conversion of amino acids) and, to a lesser extent, the count of L. dulbrecki ssp. bulgaricus. However, considering the study results, determining the effect of polysaccharides on dimethyl sulfide content is not utterly possible and requires further research.
Acetaldehyde is one of the key compounds determining the typical, fresh aroma of yogurts. It is produced by
Lactobacillus via more than one metabolic pathway, from various precursors, including lactose, valine, pyruvate, acetyl phosphate, and—most of all—threonine [
27,
28]. In the control samples made of skimmed milk, its mean content reached 2118 µg/kg and was significantly (
p < 0.001) higher than in the yogurts made of full-fat milk (83 µg/kg) (
Figure 5).
The high acetaldehyde content in milk before inoculation is probably, due to the microbiological quality of raw milk. After completed fermentation, significantly higher acetaldehyde contents were determined in the yogurts produced from skimmed milk and full-fat milk, i.e., 5084 µg/kg and 633 µg/kg, respectively. The acetaldehyde contents determined in these yogurts were significantly higher than those presented by Kaminarides et al. [
27] (180 µg/kg). The addition of the polysaccharide preparations to yogurts led to acetaldehyde content decrease, consistent with the study by Elsanhoty et al. [
17], who used 0.75% addition of OBG of unknown purity. In addition, acetaldehyde production by bacteria was significantly dependent on polysaccharide’s structure; namely, the highest increase (242%; 3145 µg/kg) in its content was noted in the yogurts with curdlan featuring a linear structure and the smallest one (122%; 126 µg/kg) in the yogurt with OBG which structure is a more complex. Also, a greater increase in acetaldehyde content was shown in the yogurts made of skimmed than full-fat milk.
After 28-day storage of yogurts, the content of acetaldehyde depended significantly (
p < 0.001) on fat concentration in milk as well as preparation type and additional level. The yogurts with OBG were greater (528%; 879 µg/kg) increase was determined in the yogurts made of full-fat than skimmed milk (188%; 558 µg/kg). In turn, in the yogurt samples with curdlan, the acetaldehyde content decreased in these made of skimmed milk (−13% on average; 276 µg/kg), but increased by 435% (1912 µg/kg) in these made of full-fat milk, which is associated with oxidative changes taking place in yogurts during storage [
31]. The presented results stay in opposition to the study by Elsanhoty et al. [
17], who demonstrated that a 0.75% addition of an OBG preparation (with unknown β-glucan concentration) led to acetaldehyde content decrease. Considering the yogurt cultures used (
L. dulbrecki ssp.
bulgaricus and
Str. termophilus), and the fact that traditional SLAB cultures do not synthesize enzymes that metabolize acetaldehyde to, i.a., ethanol, acetaldehyde content should rather increase than decrease. Nevertheless, genetically-modified strains can decrease acetaldehyde content and increase ethanol content in yogurt samples [
32]. Besides, the higher acetaldehyde content in the yogurts with OBG was, probably, due to their manufacture method (longer time of mixing the raw material and higher temperature of glucan preparation dissolution), resulting in better milk aeration before the inoculation, which enhanced aldehyde synthesis by SLAB [
27,
31].
2,3-Pentanedione and diacetyl represent ketones produced in yogurts via β-oxidation of saturated fatty acids and decarboxylation of β-keto acids. Diacetyl accumulated in yogurt is then converted to acetoin by diacetyl reductase [
32]. After completed fermentation, a significantly higher increase in diacetyl content was noted in the yogurts with OBG than in these with curdlan, with the amount of diacetyl produced being higher (
p < 0.001), the higher was the polysaccharide concentration (
Figure 5). A similar dependency was observed for 2,3-pentanedione. After 28-day storage, diacetyl content increased significantly in the yogurt samples. The highest increase in its content was determined in the yogurts made of skimmed and full-fat milk with OBG and reached, on average, 420% (358 µg/kg) and 353% (2768 µg/kg), respectively. In the respective yogurt samples with curdlan addition, the increase in its content was smaller and reached, on average, 216% (415 µg/kg) and 213% (2117 µg/kg), respectively. Regardless of the preparation used, the highest increase in diacetyl content was noted in the yogurts with 0.25% addition and the lowest one—in these with 1% addition of the polysaccharide, which indicates a significant effect of polysaccharide concentration on the potential consumption of, e.g., glucose, during diketone synthesis [
33]. The above results are consistent with those presented by Moineau-Jean et al. [
30], who showed a significant increase (ca. 300%) in diacetyl content over 38-day storage. In another research, during 28-day storage, diacetyl content decreased significantly in control yogurts and yogurts with 0.75% addition of OBG with unknown concentration [
17]. In contrast to the above-cited authors’ findings, our study results are due to different starter cultures used. Besides, as in the case of acetaldehyde, a different yogurt production method (longer heat-treatment, intensive aeration) enhanced oxidative transformations and increased diacetyl content in the samples with OBG. Worthy of notice is also the higher diacetyl content in the samples with 0.25% than 1% addition of the polysaccharide. Due to the high incompatibility of OBG and milk proteins, lower concentrations of the polysaccharide led to phase separation in the yogurts. Presumably, diacetyl synthesis proceeded mainly in whey, where the osmotic pressure is lower and, by this means, SLAB’s metabolic activity is higher. Study results also confirm that the raw material was an important source of diacetyl (buttery aroma) and that the fermentation process had a negligible effect on diacetyl content increase, which is consistent with the observations made by Routray and Mishry [
34] and confirms that certain aromas are developed in yogurts in other transformations not necessarily associated with lactic fermentation.
The metabolic activity of bacteria and biochemical transformations taking place during yogurt production or storage results in an increased acetoin content [
32]. The statistical analysis demonstrated that the acetoin content of yogurt samples was significantly affected by product type, storage time, polysaccharide addition level, and fat content in the raw material (
p < 0.01) (
Table S7). After completed fermentation, the average increase in acetoin content in the control yogurt reached 215% (2627 µg/kg), whereas, in the experimental yogurt, it was smaller and reached 147% on average. The acetoin content increase was higher in the yogurts made of skimmed than full-fat milk. Furthermore, it was higher in the yogurts with 0.25% than 1% addition of the polysaccharide, which was in line with the study concerning inulin addition [
35]. After 28-day storage, acetoin content decreased in the control samples, with a greater decrease observed in the yogurts made of skimmed milk (−56%; 1694 µg/kg) than full-fat milk (−11%; 538 µg/kg). A decreased acetoin content was determined in the yogurts made of full-fat milk with OBG addition and in these made of skimmed milk with curdlan addition; with the decrease being the greater, the higher was polysaccharide addition level. Most likely, in these products, acetoin was transformed into 2,3-butanediol by acetoin reductase. In turn, acetoin and diacetyl contents were observed to increase in the yogurt samples made of full-fat milk with curdlan addition. Probably, curdlan addition contributed to the modification in LAB metabolism due to which the higher acetoin content in the yogurts was mostly attributed to the activation of biochemical pathways enabling its synthesis from alfa-acetolactate [
32]. Our study results are consistent with works [
14], which demonstrated that diacetyl content depended on the starter culture’s polysaccharide and strain composition [
14]. Those authors also showed more enhanced diacetyl synthesis in the yogurt samples with inulin than in these with OBG. Changes in the concentrations of volatile compounds and other aroma-related compounds during storage are mainly due to the reactions in which they are synthesized or transformed into other compounds by bacterial metabolic enzymes and their losses caused by their oxidation [
36]. The literature provides incomplete data on the effect of OBG and—most of all—curdlan on the contents of various aroma compounds in yogurts. However, some studies have shown that OBG can adversely affect the content of volatiles in cheeses [
37].