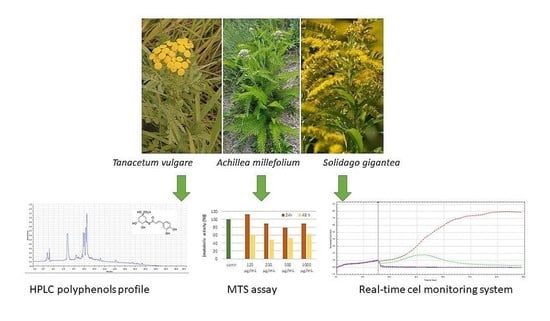
Analysis of Cytotoxicity of Selected Asteraceae Plant Extracts in Real Time, Their Antioxidant Properties and Polyphenolic Profile
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cytotoxicity Assays
2.2. Antioxidant Activity
2.3. Polyphenolic Profile
3. Materials and Methods
3.1. Materials
3.2. Extracts Preparation
3.3. Cell Cultivation
3.4. Real-Time Cell Analysis–xCELLigence System
3.5. Cytotoxicity Assay
3.6. Antioxidant Activity and Total Phenolic Compounds
3.7. HPLC Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ala, A.A.; Olotu, B.B.; Ohia, C.M.D. Assessment of cytotoxicity of leaf extracts of Andrographis paniculata and Aspilia africana on murine cells in vitro. Arch. Basic Appl. Med. 2018, 6, 61–65. [Google Scholar] [PubMed]
- García-Risco, M.R.; Mouhid, L.; Salas-Pérez, L.; López-Padilla, A.; Santoyo, S.; Jaime, L.; de Molina, A.R.; Reglero, G.; Fornari, T. Biological Activities of Asteraceae (Achillea millefolium and Calendula officinalis) and Lamiaceae (Melissa officinalis and Origanum majorana) Plant Extracts. Plant Foods Hum. Nutr. 2017, 72, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dżugan, M.; Sowa, P.; Kwaśniewska, M.; Wesołowska, M.; Czernicka, M. Physicochemical parameters and antioxidant activity of bee honey enriched with herbs. Plant Foods Hum. Nutr. 2017, 72, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Sowa, P.; Tarapatskyy, M.; Puchalski, C.; Jarecki, W.; Dżugan, M. A novel honey-based product enriched with coumarin from Melilotus flowers. J. Food Meas. Charact. 2019, 13, 1748–1754. [Google Scholar] [CrossRef] [Green Version]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [Green Version]
- Carratù, B.; Federici, E.; Gallo, F.R.; Geraci, A.; Guidotti, M.; Multari, G.; Palazzino, G.; Sanzini, E. Plants and parts of plants used in food supplements: An approach to their safety assessment. Ann. Ist. Super Sanita 2010, 46, 370–388. [Google Scholar] [CrossRef]
- Lakshmi, T.; Geetha, R.V.; Roy, A.; Aravind Kumar, S. Yarrow (Achillea millefolium Linn.) a herbal medicinal plant with broad therapeutic use—A review. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 136–141. [Google Scholar]
- Vasileva, A.M.; Iliev, I.A.; Lozanov, V.S.; Dimitrova, M.B.; Mitev, V.I.; Ivanov, I.P. In vitro study on the antitumor activity of Tanacetum vulgare L. extracts. Bulg. Chem. Commun. 2019, 51, 249–255. [Google Scholar] [CrossRef]
- Lahlou, S.; Tangi, K.C.; Lyoussi, B.; Morel, N. Vascular effects of Tanacetum vulgare L. leaf extract: In vitro pharmacological study. J. Ethnopharmacol. 2008, 120, 98–102. [Google Scholar] [CrossRef]
- Gadgoli, C.; Mishra, S.H. Preliminary screening of Achillea millefolium, Cichorium intybus and Capparis spinosa for anti-hepatotoxic activity. Fitoterapia 1995, 66, 319–323. [Google Scholar]
- Cote, H.; Boucher, M.A.; Pichette, A.; Legault, J. Anti-Inflammatory, Antioxidant, Antibiotic, and Cytotoxic Activities of Tanacetum vulgare L. Essential Oil and Its Constituents. Medicines 2017, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Devrnja, N.; Anđelković, B.; Aranđelović, S.; Radulović, S.; Soković, M.; Krstić-Milošević, D.; Ristić, M.; Ćalić, D. Comparative studies on the antimicrobial and cytotoxic activities of Tanacetum vulgare L. essential oil and methanol extracts. S. Afr. J. Bot. 2017, 111, 212–221. [Google Scholar] [CrossRef]
- Bączek, K.; Kosakowska, O.; Przybył, J.L.; Pióro-Jabrucka, E.; Costa, R.; Mondello, L.; Gniewosz, M.; Synowiec, A.; Weglarz, Z. Antibacterial and antioxidant activity of essential oils and extracts from costmary (Tanacetum balsamita L.) and tansy (Tanacetum vulgare L.). Ind. Crop. Prod. 2017, 102, 154–163. [Google Scholar] [CrossRef]
- Ivănescu, B.; Tuchiluș, C.; Corciovă, A.; Lungu, C.; Mihai, C.T.; Gheldiu, A.M.; Vlase, L. Antioxidant, antimicrobial and cytotoxic activity of Tanacetum vulgare, Tanacetum corymbosum and Tanacetum macrophyllum extracts. Farmacia 2018, 66, 282–288. [Google Scholar]
- Stojković, M.B.; Mitić, S.S.; Pavlović, J.L.; Stojanović, B.T.; Paunović, D.D. Antioxidant potential of Tanacetum vulgare L. extracts. Biol. Nyssana 2014, 5, 47–51. [Google Scholar]
- Clancy, M.V.; Zytynska, S.E.; Senft, M.; Weisser, W.W.; Schnitzler, J.P. Chemotypic variation in terpenes emitted from storage pools influences early aphid colonisation on tansy. Sci. Rep. 2016, 6, 38087. [Google Scholar] [CrossRef] [Green Version]
- Juan-Badaturuge, M.; Habtemariam, S.; Jackson, C.; Thomas, M.J.K. Antioxidant Principles of Tanacetum vulgare L. Aerial Parts. Nat. Prod. Commun. 2009, 4, 1561–1564. [Google Scholar] [CrossRef] [Green Version]
- Baretta, I.P.; Felizardo, R.A.; Bimbato, V.F.; dos Santos, M.G.; Kassuya, C.A.; Gasparotto, J.A.; da Silva, C.R.; de Oliveira, S.M.; Ferreira, J.; Andreatini, R. Anxiolytic-like effects of acute and chronic treatment with Achillea millefolium L. extract. J. Ethnopharmacol. 2012, 140, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Saeidnia, S.; Gohari, A.R.; Mokhber-Dezfuli, N.; Kiuchi, F. A review on phytochemistry and medicinal properties of the genus Achillea. DARU J. Pharm. Sci. 2011, 19, 173–186. [Google Scholar]
- Keser, S.; Celik, S.; Turkoglu, S.; Yilmaz, Ö.; Turkoglu, I. Antioxidant activity, total phenolic and flavonoid content of water and ethanol extracts from Achillea millefolium L. Turk. J. Pharm. Sci. 2013, 10, 385–392. [Google Scholar]
- El-Kalamouni, C.; Venskutonis, P.R.; Zebib, B.; Merah, O.; Raynaud, C.; Talou, T. Antioxidant and Antimicrobial Activities of the Essential Oil of Achillea millefolium L. Grown in France. Medicines 2017, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgieva, L.; Gadjalova, A.; Mihaylova, D.; Pavlov, A. Achillea millefolium L.–phytochemical profile and in vitro antioxidant activity. Int. Food Res. J. 2015, 22, 1347–1352. [Google Scholar]
- Bais, S. Review on Phytochemical and Pharmacological Activity of Yarrow (Achillea millefolium Linn). Der Pharma Chem. 2017, 9, 89–96. [Google Scholar]
- Woźniak, D.; Ślusarczyk, S.; Domaradzki, K.; Dryś, A.; Matkowski, A. Comparison of Polyphenol Profile and Antimutagenic and Antioxidant Activities in Two Species Used as Source of Solidaginis herba–Goldenrod. Chem. Biodivers. 2018, 15, e1800023. [Google Scholar] [CrossRef] [PubMed]
- Stefanic, E.; Puskadija, Z.; Stefanic, I.; Bubalo, D. Goldenrod: A valuable plant for beekeeping in north-eastern Croatia. Bee World 2003, 84, 88–92. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, antioxidant and antimicrobial activities of leaf and barkextracts of Solidago canadensis L. Ind. Crop. Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- De Barros, M.; Mota da Silva, L.; Boeing, T.; Somensi, L.B.; Cury, B.J.; de Moura Burci, L.; Santin, J.R.; de Andrade, S.F.; Monache, F.D.; Cechinel-Filho, V. Pharmacological reports about gastroprotective effects of methanolic extract from leaves of Solidago chilensis (Brazilian arnica) and its components quercitrin and afzelin in rodents. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 403–417. [Google Scholar] [CrossRef]
- Wang, Z.; Kim, J.H.; Jang, Y.S.; Kim, C.H.; Lee, J.; Lim, S.S. Anti-obesity effect of Solidago virgaurea var. gigantea extract through regulation of adipogenesis and lipogenesis pathways in high-fat diet-induced obese mice (C57BL/6N). Food Nutr. Res. 2017, 61, 1273479. [Google Scholar] [CrossRef] [Green Version]
- Toiu, A.; Vlase, L.; Vodnar, D.C.; Gheldiu, A.M.; Oniga, I. Solidago graminifolia L. Salisb. (Asteraceae) as a Valuable Source of Bioactive Polyphenols: HPLC Profile, In Vitro Antioxidant and Antimicrobial Potential. Molecules 2019, 24, 2666. [Google Scholar] [CrossRef] [Green Version]
- Leuschner, J. Anti-inflammatory, spasmolytic and diuretic effects of a commercially available Solidago gigantea Herb. extract. Arzneimittel-forschung 1995, 45, 165–168. [Google Scholar] [PubMed]
- Kołodziej, B.; Kowalski, R.; Kędzia, B. Antibacterial and antimutagenic activity of extracts aboveground parts of three Solidago species: Solidago virgaurea L.; Solidago canadensis L. and Solidago gigantea Ait. J. Med. Plants Res. 2011, 5, 6770–6779. [Google Scholar]
- Dobjanschi, L.; Păltinean, R.; Vlase, L.; Babotă, M.; Fritea, L.; Tămaș, M. Comparative phytochemical research of Solidago genus: S. graminifolia. Note, I. Flavonoids. Acta Biol. Marisiensis 2018, 1, 18–26. [Google Scholar] [CrossRef]
- Gazala, Q.; Ara, S.; Ansari, K.M.; Murtaza, I.; Qazi, H. Cytotoxicological evaluation of semi-purified extracts of some dye yielding plants of the Kashmir Valley on Normal Intestinal Cell Line (IEC-6) by MTT assay. J. Phytopharmacol. 2018, 7, 5–9. [Google Scholar]
- Kelber, O.; Bauer, R.; Kubelka, W. Phytotherapy in Functional Gastrointestinal Disorders. Dig. Dis. 2017, 35, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Zhu, Z.; Jin, L.; Chen, J.; Xie, H.; Miozzi, J.; Lei, F.; Wei, X.; Pan, J. Study on the quality evaluation of compound Danshen preparations based on the xCelligence real-time cell-based assay and pharmacodynamic authentication. Molecules 2018, 23, 2090. [Google Scholar] [CrossRef] [Green Version]
- Miłek, M.; Marcinčáková, D.; Legáth, J. Polyphenols Content, Antioxidant Activity, and Cytotoxicity Assessment of Taraxacum officinale Extracts Prepared through the Micelle-Mediated Extraction Method. Molecules 2019, 24, 1025. [Google Scholar] [CrossRef] [Green Version]
- Marcinčáková, D.; Červeňáková, N.; Miłek, M. In Vitro Evaluation of Biological Effects of Dandelion (Taraxacum officinale) Extracts. Folia Vet. 2018, 62, 36–40. [Google Scholar] [CrossRef]
- Shafiee-Kermani, F.; Grusak, M.A.; Gustafson, S.J.; Lila, M.A.; Niculescu, M.D. Lower Concentrations of Blueberry Polyphenolic-Rich Extract Differentially Alter HepG2 Cell Proliferation and Expression of Genes Related to Cell-Cycle, Oxidation and Epigenetic Machinery. J. Nutr. Disord. Ther. 2012, 3, 120. [Google Scholar] [CrossRef] [Green Version]
- Szewczyk, K.; Lewandowska, U.; Owczarek, K.; Sosnowska, D.; Gorlach, S.; Koziołkiewicz, M.; Hrabec, Z.; Hrabec, E. Influence of polyphenol extract from evening primrose (Oenothera paradoxa) seeds on proliferation of caco-2 cells and on expression, synthesis and activity of matrix metalloproteinases and their inhibitors. Pol. J. Food Nutr. Sci. 2014, 64, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Nguta, J.M.; Appiah-Opong, R.; Nyarko, A.K.; Yeboah-Manu, D.; Addo, P.G.A.; Otchere, I.D.; Kissi-Twum, A. In vitro antimycobacterial and cytotoxic data on medicinal plants used to treat tuberculosis. Data Br. 2016, 7, 1124–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirvu, L.; Stefaniu, A.; Neagu, G.; Albu, B.; Pintilie, L. In Vitro Cytotoxic and Antiproliferative Activity of Cydonia oblonga flower petals, leaf and fruit pellet ethanolic extracts. Docking simulation of the active flavonoids on anti-apoptotic protein Bcl-2. Open Chem. 2018, 16, 591–604. [Google Scholar] [CrossRef]
- Galani, B.R.T.; Sahuc, M.E.; Njayou, F.N.; Deloison, G.; Mkounga, P.; Feudjou, W.F.; Brodin, P.; Rouillé, Y.; Nkengfack, A.E.; Moundipa, P.F.; et al. Plant extracts from Cameroonian medicinal plants strongly inhibit hepatitis C virus infection in vitro. Front. Microbiol. 2015, 6, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, A.; Mohammadi-Kamalabadi, M.; Rafieian-Kopaei, M.; Amjad, L.; Salimzadeh, L. Determination of antioxidant activity, phenolic contents and antiviral potential of methanol extract of Euphorbia spinidens Bornm (Euphorbiaceae). Trop. J. Pharm. Res. 2016, 15, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Kšonžeková, P.; Mariychuk, R.; Eliašová, A.; Mudroňová, D.; Csank, T.; Király, J.; Marcinčáková, D.; Pistl, J.; Tkáčiková, L. In vitro study of biological activities of anthocyanin-rich berry extracts on porcine intestinal epithelial cells. J. Sci. Food Agric. 2016, 96, 1093–1100. [Google Scholar] [CrossRef]
- Pagliacci, M.C.; Spinozzi, F.; Miglioratii, G.; Gumi, F.; Smacchia, M.; Grignani, F.; Riccardi, C.; Nicoletti, I. Genistein inhibits tumour cell growth in vitro but enhances mitochondrial reduction of tetrazolium salts: A further pitfall in the use of the MTT assay for evaluating cell growth and survival. Eur. J. Cancer 1993, 29A, 1573–1577. [Google Scholar] [CrossRef]
- Gomes, D.B.; Zanchet, B.; Locateli, G.; Benvenutti, R.C.; Vechia, C.A.D.; Schönell, A.P.; Diel, K.A.P.; Zilli, G.A.L.; Miorando, D.; Ernetti, J.; et al. Antiproliferative potential of solidagenone isolated of Solidago chilensis. Rev. Bras. Farmacogn. 2018, 28, 703–709. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Hosseini, F.; Ahmadi, A.; Mozafari, M.; Amjadi, I. Antiproliferative activity of hypericum perforatum, achillea millefolium, and aloe vera in interaction with the prostatic activity of CD82. Rep. Biochem. Mol. Biol. 2019, 8, 260–268. [Google Scholar]
- Mureşan, M.; Benedec, D.; Vlase, L.; Oprean, R.; Toiu, A.; Oniga, I. Screening of polyphenolic compounds, antioxidant and antimicrobial properties of Tanacetum vulgare from Transylvania. Studia Univ. Babes Bolyai Chem. 2015, 60, 127–138. [Google Scholar]
- Fraisse, D.; Felgines, C.; Texier, O.; Lamaison, J.-L. Caffeoyl Derivatives: Major Antioxidant Compounds of Some Wild Herbs of the Asteraceae Family. Food Nutr. Sci. 2011, 2, 181–192. [Google Scholar] [CrossRef]
- Tadić, V.; Arsić, I.; Zvezdanović, J.; Zugić, A.; Cvetković, D.; Pavkov, S. The estimation of the traditionally used yarrow (Achillea millefolium L. Asteraceae) oil extracts with anti-inflamatory potential in topical application. J. Ethnopharmacol. 2017, 199, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Pereira, C.; Tzortzakis, N.; Barros, N.; Ferreira, I.C.F.R. Nutritional Value and Bioactive Compounds Characterization of Plant Parts From Cynara cardunculus L. (Asteraceae) Cultivated in Central Greece. Front. Plant Sci. 2018, 9, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Zhou, J.; Shi, Y.; Karaisz, K. Identifi cation of Antioxidative Ingredients from Feverfew (Tanacetum parthenium) Extract Substantially free of Parthenolide and other Alpha- Unsaturated Gamma-Lactones. Open J. Anal. Bioanal. Chem. 2019, 3, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Marksa, M.; Zymone, K.; Ivanauskas, L.; Radušienė, J.; Pukalskas, A.; Raudone, L. Antioxidant profiles of leaves and inflorescences of native, invasive and hybrid Solidago species. Ind. Crop. Prod. 2020, 145, 112123. [Google Scholar] [CrossRef]
- Campos, M.G.; Markham, K.R. Structure information from HPLC and on-line measured absorption spectra: Flavones, Flavonols and Phenolic Acids. In Imprensa da Universidade de Coimbra, Coimbra; Coimbra University Press: Coimbra, Portugal, 2007. [Google Scholar]
- Lin, L.-Z.; Harnly, J.M. A Screening Method for the Identification of Glycosylated Flavonoids and Other Phenolic Compounds Using a Standard Analytical Approach for All Plant Materials. J. Agric. Food Chem. 2007, 55, 1084–1096. [Google Scholar] [CrossRef] [Green Version]
- Fraisse, D.; Degerine-Roussel, A.; Bred, A.; Ndoye, S.F.; Vivier, M.; Felgines, C.; Senejoux, F. A Novel HPLC Method for Direct Detection of Nitric Oxide Scavengers from Complex Plant Matrices and Its Application to Aloysia triphylla Leaves. Molecules 2018, 23, 1574. [Google Scholar] [CrossRef] [Green Version]
- Raudone, L.; Vilkickyte, G.; Pitkauskaite, L.; Raudonis, R.; Vainoriene, R.; Motiekaityte, V. Antioxidant Activities of Vaccinium vitis-idaea L. Leaves within Cultivars and Their Phenolic Compounds. Molecules 2019, 24, 844. [Google Scholar] [CrossRef] [Green Version]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. a thorough study of reactivity of various compound classes towards the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Young, L.; Sung, J.; Stacey, G.; Masters, J.R. Detection of Mycoplasma in cell cultures. Nat. Protoc. 2010, 5, 929–934. [Google Scholar] [CrossRef]
- Miłek, M.; Marcinčáková, D.; Csank, T.; Kšonžeková, P.; Falis, M.; Legáth, J.; Pistl, J. Real-time monitoring of cadmium toxicity in rabbit kidney cells. Acta Vet. Brno 2015, 84, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Marcinčáková, D.; Schusterová, P.; Mudroňová, D.; Csank, T.; Falis, M.; Fedorová, M.; Marcinčák, S.; Hus, K.K.; Legáth, J. Impact of zinc sulfate exposition on viability, proliferation and cell cycle distribution of epithelial kidney cells. Polish J. Environ. Stud. 2019, 28, 3279–3286. [Google Scholar] [CrossRef]

| Concentration of Extracts (µg/mL) | ||||||
|---|---|---|---|---|---|---|
| 125 µg/mL | 250 µg/mL | 500 µg/mL | 1000 µg/mL | Control | ||
| Tansy leaves | ||||||
| RTCA | CI | 0.13 ± 0.01 *** | 0.07 ± 0.01 *** | 0.05 ± 0.01 *** | 0.08 ± 0.02 *** | 4.07 ± 0.74 |
| A (%) | 3.28 ± 0.28 *** | 1.64 ± 0.14 *** | 1.31 ± 0.14 *** | 1.88 ± 0.51 *** | 100 ± 18.23 | |
| MTS | MA (%) | 42.95 ± 1.54 *** | 49.25 ± 0.00 *** | 66.42 ± 2.45 *** | 82.15 ± 4.05 *** | 100 ± 0.00 |
| Yarrow leaves | ||||||
| RTCA | CI | 1.15 ± 0.07 *** | 0.72 ± 0.09 *** | 0.59 ± 0.07 *** | 0.64 ± 0.11 *** | 4.07 ± 0.74 |
| A (%) | 28.34 ± 1.64 *** | 17.61 ± 2.09 *** | 14.41 ± 1.60 *** | 15.72 ± 2.70 *** | 100 ± 18.23 | |
| MTS | MA (%) | 46.03 ± 1.77 *** | 50.45 ± 1.28 *** | 59.51 ± 1.44 *** | 76.47 ± 1.71 *** | 100 ± 0.00 |
| Goldenrod leaves | ||||||
| RTCA | CI | 9.18 ± 2.78 *** | 3.74 ± 0.62 | 0.08 ± 0.01 *** | 0.07 ± 0.01 *** | 4.07 ± 0.74 |
| A (%) | 225.55 ± 68.26 *** | 91.81 ± 15.31 | 1.88 ± 0.28 *** | 1.64 ± 0.14 *** | 100 ± 18.23 | |
| MTS | MA (%) | 61.22 ± 3.27 *** | 49.59 ± 1.67 *** | 57.32 ± 2.02 *** | 76.81 ± 2.80 *** | 100 ± 0.00 |
| Goldenrod flowers | ||||||
| RTCA | CI | 8.60 ± 2.56 *** | 2.42 ± 0.23 | 0.31 ± 0.10 *** | 0.12 ± 0.03 *** | 4.07 ± 0.74 |
| A (%) | 211.38 ± 62.87 *** | 59.46 ± 5.54 *** | 7.70 ± 2.56 *** | 3.03 ± 0.79 *** | 100 ± 18.23 | |
| MTS | MA (%) | 60.12 ± 4.53 *** | 47.74 ± 2.53 *** | 51.44 ± 1.20 *** | 62.04 ± 0.66 *** | 100 ± 0.00 |
| Extract | Antioxidant Capacity | ||
|---|---|---|---|
| TPC (mg GAE/g) | DPPH Reduction (μmol TE/g) | FRAP (μmol TE/g) | |
| Tansy leaves | 41.35 ± 1.48 a | 257.17 ± 16.69 a | 243.21 ± 3.02 a |
| Yarrow leaves | 15.86 ± 2.73 d | 92.19 ± 4.92 c | 84.17 ± 6.04 d |
| Goldenrod leaves | 50.99 ± 1.63 c | 272.47 ± 5.68 a | 272.25 ± 19.94 c |
| Goldenrod flowers | 32.58 ± 0.94 b | 192.82 ± 4.11 b | 192.76 ± 5.59 b |
| Compound (mg/g) | Retention Time (min) | Tansy Leaves | Yarrow Leaves | Goldenrod Leaves | Goldenrod Flowers |
|---|---|---|---|---|---|
| Chlorogenic acid der | 3.27 | n.d. | 1.92 ± 0.05 | 1.24 ± 0.02 | 0.72 ± 0.01 |
| 6.99 | 8.41 ± 0.11 | 1.95 ± 0.02 | 0.46 ± 0.02 | 1.27 ± 0.04 | |
| 8.80 | n.d. | 0.33 ± 0.01 | n.d. | n.d. | |
| 10.14 | n.d. | 0.26 ± 0.01 | n.d. | n.d. | |
| Chlorogenic acid | 6.25 | 37.64 ± 0.24 | 23.22 ± 0.80 | 8.92 ± 0.50 | 11.08 ± 0.13 |
| Caffeic acid | 2.91 | 1.33 ± 0.12 | 1.58 ± 0.02 | 2.79 ± 0.01 | 1.27 ± 0.02 |
| Caffeic acid der | 2.58 | n.d. | 0.89 ± 0.02 | n.d. | 1.92 ± 0.01 |
| Ferulic acid | 4.50 | 14.22 ± 0.13 | 0.27 ± 0.01 | n.d. | n.d. |
| Ferulic acid der | 5.33 | n.d. | 0.21 ± 0.01 | n.d. | n.d. |
| Unknown phenolic acid | 2.20 | 4.92 ± 0.05 | n.d. | n.d. | n.d. |
| Hydroxycinnamic acid derivatives total | 66.52 ± 27.32 | 28.74 ± 10.56 | 12.17 ± 4.69 | 15.54 ± 6.29 | |
| Rutin | 7.85 | n.d. | n.d. | 1.25 ± 0.03 | 0.43 ± 0.02 |
| Hyperoside | 8.31 | 6.67 ± 0.60 | n.d. | 4.40 ± 0.50 | 7.70 ± 0.09 |
| Quercetin Glycoside der | 9.50 | 1.63 ± 0.03 | n.d. | 5.98 ± 0.02 | 7.95 ± 0.09 |
| 10.12 | n.d. | n.d. | 34.54 ± 0.10 | 10.60 ±0.03 | |
| Luteolin | 13.91 | 1.17 ± 0.01 | 0.19 ± 0.01 | <LOQ | n.d. |
| Luteolin der | 13.49 | 0.85 ± 0.02 | n.d. | n.d. | n.d. |
| Apigenin | 15.70 | 0.78 ± 0.02 | n.d. | n.d. | 0.16 ± 0.01 |
| Apigenin der | 7.59 | 2.52 ± 0.03 | 0.47 ± 0.05 | n.d. | n.d. |
| Flavonoid derivatives total | 13.62 ± 4.89 | 0.66 ± 0.20 | 46.17 ± 15.46 | 26.84 ± 4.37 | |
| Total polyphenols | 80.14 | 29.40 | 58.34 | 48.12 |
Sample Availability: Samples of the plant extracts are available from the authors for the limited time. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowa, P.; Marcinčáková, D.; Miłek, M.; Sidor, E.; Legáth, J.; Dżugan, M. Analysis of Cytotoxicity of Selected Asteraceae Plant Extracts in Real Time, Their Antioxidant Properties and Polyphenolic Profile. Molecules 2020, 25, 5517. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25235517
Sowa P, Marcinčáková D, Miłek M, Sidor E, Legáth J, Dżugan M. Analysis of Cytotoxicity of Selected Asteraceae Plant Extracts in Real Time, Their Antioxidant Properties and Polyphenolic Profile. Molecules. 2020; 25(23):5517. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25235517
Chicago/Turabian StyleSowa, Patrycja, Dana Marcinčáková, Michał Miłek, Ewelina Sidor, Jaroslav Legáth, and Małgorzata Dżugan. 2020. "Analysis of Cytotoxicity of Selected Asteraceae Plant Extracts in Real Time, Their Antioxidant Properties and Polyphenolic Profile" Molecules 25, no. 23: 5517. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25235517