Transport of N-CD and Pre-Sorbed Pb in Saturated Porous Media
Abstract
:1. Introduction
2. Results and Discussion
2.1. Properties of N-CDs
2.2. N-CD Aggregation and N-CD-Quartz Interactions
2.3. Pb Adsorption on N-CD and Quartz Surface
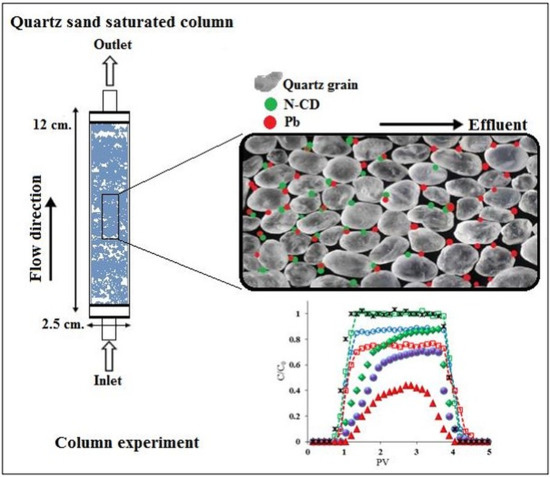
2.4. Impact of N-Functionalization on the CD Transport and Pb Transport
2.5. Effects of Environmental Factors on N-CDs and r-Pb Transport
2.5.1. Effects of Ionic Strength and Cation Type
2.5.2. Effects of pH
3. Material and Methods
3.1. Preparation of N-CDs
3.2. Characterization of N-CDs
3.3. Porous Media
3.4. Column Transport Experiments
3.5. Batch Experiments
3.6. Derjaguin, Landau, Vervey, and Overbeek (DLVO) Theory
4. Conclusions and Prospective
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.; Miao, P.; Zhou, W.; Gong, X.; Zhao, X. N-doped carbon-dots for luminescent solar concentrators. J. Mater. Chem. A 2017, 5, 21452–21459. [Google Scholar] [CrossRef]
- Semeniuk, M.; Yi, Z.; Poursorkhabi, V.; Tjong, J.; Jaffer, S.; Lu, Z.-H.; Sain, M. Future Perspectives and Review on Organic Carbon Dots in Electronic Applications. ACS Nano 2019, 13, 6224–6255. [Google Scholar] [CrossRef]
- Jlassi, K.; Eid, K.; Sliem, M.H.; Abdullah, A.M.; Chehimi, M.; Krupa, I. Rational synthesis, characterization, and application of environmentally friendly (polymer-carbon dot) hybrid composite film for fast and efficient UV-assisted Cd2+ removal from water. Environ. Sci. Eur. 2020, 32, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef] [Green Version]
- Xie, R.; Jin, Y.; Chen, Y.; Jiang, W. The importance of surface functional groups in the adsorption of copper onto walnut shell derived activated carbon. Water Sci. Technol. 2017, 76, 3022–3034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Gómez-Serrano, V.; Alvarez, P.M.; Alvim-Ferraz, M.C.; Dias, J.M. Activated carbon modifications to enhance its water treatment applications. An overview. J. Hazard. Mater. 2011, 187, 1–23. [Google Scholar] [CrossRef]
- Shen, W.; Fan, W. Nitrogen-containing porous carbons: Synthesis and application. J. Mater. Chem. A 2013, 1, 999–1013. [Google Scholar] [CrossRef]
- Li, S.; Liu, H.; Gao, R.; Abdurahman, A.; Dai, J.; Zeng, F. Aggregation kinetics of microplastics in aquatic environment: Complex roles of electrolytes, pH, and natural organic matter. Environ. Pollut. 2018, 237, 126–132. [Google Scholar] [CrossRef]
- Yu, C.; Guo, X.; Gao, X.; Yu, Z.; Jiang, J. Transport of graphene quantum dots (GQDs) in saturated porous media. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124418. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Gao, B.; Li, Y.; Sun, H.; Wang, M. Transport of N-doped graphene in saturated porous media. Chem. Eng. J. 2019, 360, 24–29. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Jiang, X.; Lu, Y.; Fan, W.; Huo, M.; Crittenden, J. Cotransport of graphene oxide and Cu(II) through saturated porous media. Sci. Total. Environ. 2016, 550, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Jiang, Y.; Tan, Y.; Meng, X.; Sun, H.; Wang, N. Co-transport of graphene oxide and heavy metal ions in surface-modified porous media. Chemosphere 2019, 218, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kamrani, S.; Rezaei, M.; Kord, M.; Baalousha, M. Transport and retention of carbon dots (CDs) in saturated and unsaturated porous media: Role of ionic strength, pH, and collector grain size. Water Res. 2018, 133, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Kamrani, S.; Rezaei, M.; Kord, M.; Baalousha, M. Co-transport and remobilization of Cu and Pb in quartz column by carbon dots. Sci. Total. Environ. 2018, 626, 995–1004. [Google Scholar] [CrossRef]
- Dhenadhayalan, N.; Lin, K.-C.; Suresh, R.; Ramamurthy, P. Unravelling the Multiple Emissive States in Citric-Acid-Derived Carbon Dots. J. Phys. Chem. C 2016, 120, 1252–1261. [Google Scholar] [CrossRef]
- Molaei, M.J. A review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence. Talanta 2019, 196, 456–478. [Google Scholar] [CrossRef]
- Beiraghi, A.; Najibi-Gehraz, S.A. Purification and Fractionation of Carbon Dots using pH controlled Cloud Point Extraction Technique. J. Nanostruct. 2020, 10, 107–118. [Google Scholar]
- Guo, B.; Liu, Q.; Chen, E.; Zhu, H.; Fang, L.; Gong, J.R. Controllable N-Doping of Graphene. Nano Lett. 2010, 10, 4975–4980. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, C.Y.; Chiu, P.W. Controllable graphene N-doping with ammonia plasma. Appl. Phys. Lett. 2010, 96, 133110–133113. [Google Scholar] [CrossRef]
- Xu, X.; Gao, F.; Bai, X.; Liu, F.; Kong, W.; Li, M. Tuning the photoluminescence of graphene quantum dots by photochemical doping with nitrogen. Materials 2017, 10, 1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issa, M.A.; Abidin, Z.Z.; Sobri, S.; Rashid, S.A.; Mahdi, M.A.; Ibrahim, N.A.; Pudza, M.Y. Facile Synthesis of Nitrogen-Doped Carbon Dots from Lignocellulosic Waste. Nanomaterials 2019, 9, 1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, Y.; Park, S.-J.; Zhang, Y.; Kim, T.; Chae, S.; Park, M.; Kim, H.-Y. One-step synthesis of robust nitrogen-doped carbon dots: Acid-evoked fluorescence enhancement and their application in Fe3+ detection. J. Mater. Chem. A 2015, 3, 17747–17754. [Google Scholar] [CrossRef]
- Wang, T.; Chen, G.; Li, L.; Wu, Y. Highly Fluorescent Green Carbon Dots as a Fluorescent Probe for Detecting Mineral Water pH. Sensors 2019, 19, 3801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, L.; Li, Z.-T.; Lu, H.; Yu, S.; Ding, X.; Xu, K.; Li, Z.-T.; Zhang, J.Z. Highly Photoluminescent and Stable N-Doped Carbon Dots as Nanoprobes for Hg2+ Detection. Nanomaterials 2018, 8, 900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Liu, L.; Gao, B.; Muñoz-Carpena, R.; Zhang, M.; Chen, H.; Zhou, Z.; Wang, H. Aggregation Kinetics of Graphene Oxides in Aqueous Solutions: Experiments, Mechanisms, and Modeling. Langmuir 2013, 29, 15174–15181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, X.; Huang, Q.; Walker, S.L.; Cai, P. Interactions of pathogens Escherichia coli and Streptococcus suis with clay minerals. Appl. Clay Sci. 2012, 69, 37–42. [Google Scholar] [CrossRef]
- Wu, X.; Lyu, X.; Li, Z.; Gao, B.; Zeng, X.; Wu, J.; Sun, Y. Transport of polystyrene nanoplastics in natural soils: Effect of soil properties, ionic strength and cation type. Sci. Total Environ. 2020, 707, 136065. [Google Scholar] [CrossRef]
- Kim, H.; Walker, S.L.; Bradford, S.A. Macromolecule mediated transport and retention of Escherichia coli O157:H7 in saturated porous media. Water Res. 2010, 44, 1082–1093. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Panneerselvam, P.; Marieeswaran, M.; Kothalam, R.; Perumal, P.; Muppidathi, M. A green synthetic route for the surface-passivation of carbon dots as an effective multifunctional fluorescent sensor for the recognition and detection of toxic metal ions from aqueous solution. Anal. Methods 2019, 11, 490–506. [Google Scholar] [CrossRef]
- Konkena, B.; Vasudevan, S. Spectral Migration of Fluorescence in Graphene Oxide Aqueous Dispersions: Evidence for Excited-State Proton Transfer. J. Phys. Chem. Lett. 2013, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Syamantak, K.; Navneet, C.V.; Prashant, G.; Sanjhal, J.; Souvik, G.; Nandi, C.K. Mechanistic Insight into the Carbon Dots: Protonation induced Photoluminescence. J. Mater. Sci. Eng. 2018, 7, 3. [Google Scholar] [CrossRef]
- Xu, D.; Tan, X.; Chen, C.; Wang, X. Removal of Pb(II) from aqueous solution by oxidized multiwalled carbon nanotubes. J. Hazard. Mater. 2008, 154, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Thamer, B.M.; Aldalbahi, A.; Moydeen, M.; Al-Enizi, A.M.; El-Hamshary, H.; El-Newehy, M.H. Fabrication of functionalized electrospun carbon nanofibers for enhancing lead-ion adsorption from aqueous solutions. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gao, B.; Wu, L.; Muñoz-Carpena, R.; Huang, Q. Effect of solution chemistry on multi-walled carbon nanotube deposition and mobilization in clean porous media. J. Hazard. Mater. 2012, 231, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.; Galan, M.C. Fluorescent carbon dots from mono- and polysaccharides: Synthesis, properties and applications. Beilstein J. Org. Chem. 2017, 13, 675–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Tong, M.; Lu, R.; Kim, H. Transport and deposition of ZnO nanoparticles in saturated porous media. Colloids Surf. A Physicochem. Eng. Asp. 2012, 401, 29–37. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, L.; Chen, W. Transport of graphene oxide nanoparticles in saturated sandy soil. Environ. Sci. Process. Impacts 2014, 16, 2268–2277. [Google Scholar] [CrossRef]
- Mukherjee, R.; Bhunia, P.; De, S. Impact of graphene oxide on removal of heavy metals using mixed matrix membrane. Chem. Eng. J. 2016, 292, 284–297. [Google Scholar] [CrossRef]
- Bradford, S.A.; Torkzaban, S.; Walker, S.L. Coupling of physical and chemical mechanisms of colloid straining in saturated porous media. Water Res. 2007, 41, 3012–3024. [Google Scholar] [CrossRef]
- Fan, W.; Jiang, X.; Yang, W.; Geng, Z.; Huo, M.; Liu, Z.; Zhou, H. Transport of graphene oxide in saturated porous media: Effect of cation composition in mixed Na-Ca electrolyte systems. Sci. Total. Environ. 2015, 511, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, X.; Meng, X.; Li, Y.; Zhu, W. Release and transport of Pb(II) adsorbed on graphene oxide under alkaline conditions in a saturated sand column. J. Hazard. Mater. 2019, 377, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Deng, S.; Yu, G.; Huang, J. Efficient removal of Cu(II), Pb(II), Cr(VI) and As(V) from aqueous solution using an aminated resin prepared by surface-initiated atom transfer radical polymerization. Chem. Eng. J. 2010, 165, 751–757. [Google Scholar] [CrossRef]
- Terracciano, A.; Zhang, J.; Christodoulatos, C.; Wu, F.; Meng, X. Adsorption of Ca2+ on single layer graphene oxide. J. Environ. Sci. 2017, 57, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Baalousha, M.; Lead, J.R. Rationalizing Nanomaterial Sizes Measured by Atomic Force Microscopy, Flow Field-Flow Fractionation, and Dynamic Light Scattering: Sample Preparation, Polydispersity, and Particle Structure. Environ. Sci. Technol. 2012, 46, 6134–6142. [Google Scholar] [CrossRef] [PubMed]
- Torkzaban, S.; Kim, Y.; Mulvihill, M.; Wan, J.; Tokunaga, T.K. Transport and deposition of functionalized CdTe nanoparticles in saturated porous media. J. Contam. Hydrol. 2010, 118, 208–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, J.N.; Elimelech, M. Colloid mobilization and transport in groundwater. Colloids Surf. A Physicochem. Eng. Asp. 1996, 107, 1–56. [Google Scholar] [CrossRef]
- Lead, J.R.; Muirhead, D.; Gibson, C.T. Characterization of Freshwater Natural Aquatic Colloids by Atomic Force Microscopy (AFM). Environ. Sci. Technol. 2005, 39, 6930–6936. [Google Scholar] [CrossRef]
- Lead, J.R.; Wilkinson, K.J. Aquatic Colloids and Nanoparticles: Current Knowledge and Future Trends. Environ. Chem. 2006, 3, 159–171. [Google Scholar] [CrossRef]
- Gibson, C.T.; Turner, I.J.; Roberts, C.J.; Lead, J.R. Quantifying the Dimensions of Nanoscale Organic Surface Layers in Natural Waters. Environ. Sci. Technol. 2007, 41, 1339–1344. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






| pH | IS (mM NaCl) | IS (mM CaCl2) | N-CDs Zeta Potential-mV (±2.5) | CDs Zeta Potential-mV (±2.5) | N-CD Hydrodynamic Diameter-nm (±5) | CD Hydrodynamic Diameter-nm (±5) | Quartz Grains Zeta Potential-mV (±2.5) |
|---|---|---|---|---|---|---|---|
| 4 | 1 | 0 | −21.6 | −24.2 | 44.3 | 39.3 | −40 |
| 6 | 1 | 0 | −26.2 | −29.6 | 30.7 | 24.6 | −65 |
| 9 | 1 | 0 | −33.5 | −38.6 | 19.4 | 17.5 | −80 |
| 6 | 50 | 0 | −21.5 | −25.1 | 67.1 | 64.9 | −51.6 |
| 6 | 100 | 0 | −15.8 | −19.2 | 75.2 | 71.3 | −44.6 |
| 4 | 0 | 1 | −14.5 | −16.9 | 51.1 | 45.4 | −37.5 |
| 9 | 0 | 1 | −28.2 | −33.8 | 25.5 | 21.3 | −75.3 |
| 6 | 0 | 1 | −21 | −26.3 | 36.3 | 30.8 | −61.2 |
| 6 | 0 | 50 | −10.2 | −12 | 121.5 | 113.6 | −45.1 |
| 6 | 0 | 100 | −8.6 | −9.1 | 136.6 | 128.2 | −37.2 |
| Materials | Exp. Conditions | Recovered Nanoparticles (%) | Total Effluent Pb (%) | Recovered Pb after Nanoparticles Injection (%) |
|---|---|---|---|---|
| N-CD (50mg/L, Pb: 10 mg/L) | 1 mM NaCl, pH 6 | 93.3 | 63.1 | 56.9 |
| 1 mM CaCl2, pH 6 | 91.1 | 58.2 | 53.4 | |
| 50 mM NaCl, pH 6 | 76.4 | 39.7 | 33.5 | |
| 50 mM CaCl2, pH 6 | 62.7 | 33.1 | 28.3 | |
| 100 mM NaCl, pH 6 | 65.2 | 27.8 | 21.6 | |
| 100 mM CaCl2, pH 6 | 48.5 | 16.1 | 11.3 | |
| 1 mM NaCl, pH 4 | 82.6 | 27.1 | 20.9 | |
| 1 mM CaCl2, pH 4 | 74.4 | 13.5 | 8.7 | |
| 1 mM NaCl, pH 9 | 98.2 | 31.7 | 25.5 | |
| 1 mM CaCl2, pH 9 | 98.4 | 17.9 | 13.1 | |
| Carbon Dot (CD: 50 mg/L) | 1 mM NaCl, Ph 6 | 96.1 | 56.8 | 50.6 |
| 1 mM CaCl2, pH 6 | 94.6 | 51.4 | 46.6 | |
| Effluent Pb (before nanoparticles injection) | 1 mM NaCl, pH 6 | - | 6.2 | - |
| Effluent Pb (before nanoparticles injection) | 1 mM CaCl2, pH 6 | - | 4.8 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamrani, S.; Amiri, V.; Kamrani, M.; Baalousha, M. Transport of N-CD and Pre-Sorbed Pb in Saturated Porous Media. Molecules 2020, 25, 5518. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25235518
Kamrani S, Amiri V, Kamrani M, Baalousha M. Transport of N-CD and Pre-Sorbed Pb in Saturated Porous Media. Molecules. 2020; 25(23):5518. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25235518
Chicago/Turabian StyleKamrani, Salahaddin, Vahab Amiri, Mosleh Kamrani, and Mohammed Baalousha. 2020. "Transport of N-CD and Pre-Sorbed Pb in Saturated Porous Media" Molecules 25, no. 23: 5518. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25235518