Discrimination of Cultivated Regions of Soybeans (Glycine max) Based on Multivariate Data Analysis of Volatile Metabolite Profiles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Profiling of Total Volatile Compounds in Soybeans
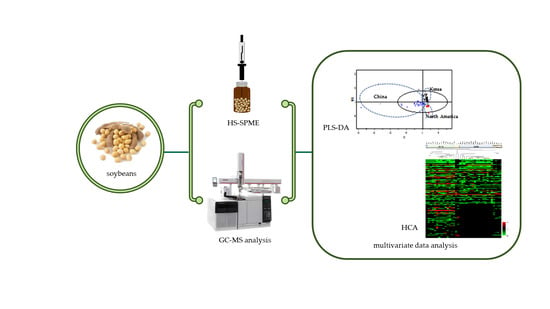
2.2. Discrimination of Soybeans by Different Geographical Origins
3. Materials and Methods
3.1. Materials
3.2. Extraction of Volatile Metabolites Using SPME
3.3. GC-MS Analysis
3.4. Identification and Quantification of Volatile Metabolites
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Singh, G. The Soybean: Botany, Production and Uses; CABI: Wallingford, UK, 2010; Volume 1, pp. 1–7. [Google Scholar]
- National Agricultural Products Quality Management Service. Available online: http://www.naqs.go.kr/eng/contents/contents.do?menuId=MN20581 (accessed on 15 October 2019).
- Kaplan, L. What is the Origin of the Common Bean? Econ. Bot. 1981, 35, 240–254. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, L.; Liu, B.; Wang, Z.; Jin, Z.; Sun, H. The Genetic Diversity of Cultivated Soybean Grown in China. Appl. Genet. 2004, 108, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Pongsuwan, W.; Fukusaki, E.; Bamba, T.; Yonetani, T.; Yamahara, T.; Kobayashi, A. Prediction of Japanese Green Tea Ranking by Gas chromatography/mass Spectrometry-Based Hydrophilic Metabolite Fingerprinting. J. Agric. Food Chem. 2007, 55, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Boulanger, R.; Dussert, S.; Ribeyre, F.; Berthiot, L.; Descroix, F.; Joët, T. Climatic Factors Directly Impact the Volatile Organic Compound Fingerprint in Green Arabica Coffee Bean as Well as Coffee Beverage Quality. Food Chem. 2012, 135, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Caruso, G.; Giunti, G.; Cuzzola, A.; Saba, A.; Raffaelli, A.; Gucci, R. Changes in Olive Oil Volatile Organic Compounds Induced by Water Status and Light Environment in Canopies of Olea Europaea, L. Trees. J. Sci. Food Agric. 2015, 95, 2473–2481. [Google Scholar] [CrossRef]
- Grieshop, C.M.; Fahey, G.C. Comparison of Quality Characteristics of Soybeans from Brazil, China, and the United States. J. Agric. Food Chem. 2001, 49, 2669–2673. [Google Scholar] [CrossRef]
- Cherry, J.H.; Bishop, L.; Hasegawa, P.M.; Lefflert, H. Differences in the Fatty Acid Composition of Soybean Seed Produced in Northern and Southern Areas of the USA. Phytochemistry 1985, 24, 237–241. [Google Scholar] [CrossRef]
- Min, S.; Yu, Y.; Yoo, S.; Martin, S.S. Effect of Soybean Varieties and Growing Locations on the Flavor of Soymilk. J. Food Sci. 2005, 70, C1–C11. [Google Scholar] [CrossRef]
- Lee, K.; Shibamoto, T. Antioxidant Properties of Aroma Compounds Isolated from Soybeans and Mung Beans. J. Agric. Food Chem. 2000, 48, 4290–4293. [Google Scholar] [CrossRef]
- Boué, S.M.; Shih, B.Y.; Carter-Wientjes, C.H.; Cleveland, T.E. Identification of Volatile Compounds in Soybean at various Developmental Stages using Solid Phase Microextraction. J. Agric. Food Chem. 2003, 51, 4873–4876. [Google Scholar] [CrossRef]
- Dings, L.; Aprea, E.; Odake, S. Comparison of Volatile Flavour Profiles of Kidney Beans and Soybeans by GC-MS and PTR-MS. Food Sci. Technol. Res. 2005, 11, 63–70. [Google Scholar]
- Oldiges, M.; Lütz, S.; Pflug, S.; Schroer, K.; Stein, N.; Wiendahl, C. Metabolomics: Current State and Evolving Methodologies and Tools. Appl. Microbiol. Biotechnol. 2007, 76, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Cubero-Leon, E.; Peñalver, R.; Maquet, A. Review on Metabolomics for Food Authentication. Food Res. Int. 2014, 60, 95–107. [Google Scholar] [CrossRef]
- Kopka, J. Current Challenges and Developments in GC–MS Based Metabolite Profiling Technology. J. Biotechnol. 2006, 124, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Kruger, N.; Ratcliffe, R. Metabolite Fingerprinting and Profiling in Plants using NMR. J. Exp. Bot. 2004, 56, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Jung, Y.; Song, B.; Bong, Y.; Lee, K.; Hwang, G. Discrimination of Cabbage (Brassica Rapa ssp. Pekinensis) Cultivars Grown in Different Geographical Areas using 1H NMR-Based Metabolomics. Food Chem. 2013, 137, 68–75. [Google Scholar] [CrossRef]
- Son, H.; Hwang, G.; Kim, K.M.; Ahn, H.; Park, W.; Van Den Berg, F.; Hong, Y.; Lee, C. Metabolomic Studies on Geographical Grapes and their Wines using 1H NMR Analysis Coupled with Multivariate Statistics. J. Agric. Food Chem. 2009, 57, 1481–1490. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, J.; Kwon, J.; Lee, K.; Ryu, D.H.; Hwang, G. Discrimination of the Geographical Origin of Beef by 1H NMR-Based Metabolomics. J. Agric. Food Chem. 2010, 58, 10458–10466. [Google Scholar] [CrossRef]
- Lee, E.M.; Park, S.J.; Lee, J.; Lee, B.M.; Shin, B.K.; Kang, D.J.; Choi, H.; Kim, Y.; Lee, D.Y. Highly Geographical Specificity of Metabolomic Traits among Korean Domestic Soybeans (Glycine Max). Food Res. Int. 2019, 120, 12–18. [Google Scholar] [CrossRef]
- Lee, B.; Zhou, Y.; Lee, J.S.; Shin, B.K.; Seo, J.; Lee, D.; Kim, Y.; Choi, H. Discrimination and Prediction of the Origin of Chinese and Korean Soybeans using Fourier Transform Infrared Spectrometry (FT-IR) with Multivariate Statistical Analysis. PLoS ONE 2018, 13, e0196315. [Google Scholar] [CrossRef]
- Ye, N.; Zhang, L.; Gu, X. Discrimination of Green Teas from Different Geographical Origins by using HS-SPME/GC–MS and Pattern Recognition Methods. Food Anal. Methods 2012, 5, 856–860. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, I.H.; Lee, K.E.; Kim, Y. The Compositions of Volatiles and Aroma-Active Compounds in Dried Omija Fruits (Schisandra Chinensis Baillon) According to the Cultivation Areas. J. Agric. Food Chem. 2011, 59, 8338–8346. [Google Scholar] [CrossRef] [PubMed]
- Aliferis, K.A.; Tarantilis, P.A.; Harizanis, P.C.; Alissandrakis, E. Botanical Discrimination and Classification of Honey Samples Applying Gas chromatography/mass Spectrometry Fingerprinting of Headspace Volatile Compounds. Food Chem. 2010, 121, 856–862. [Google Scholar] [CrossRef]
- Markley, K.S.; Goss, W.H. Soybean Chemistry and Technology; Chemical Publishing Company: Palm Springs, CA, USA, 1944; pp. 15–19. [Google Scholar]
- Rice, R.; Wei, L.; Steinberg, M.; Nelson, A. Effect of Enzyme Inactivation on the Extracted Soybean Meal and Oil. J. Am. Oil Chem. Soc. 1981, 58, 578. [Google Scholar] [CrossRef]
- Arai, S.; Noguchi, M.; Kaji, M.; Kato, H.; Fujimaki, M. N-Hexanal and some Volatile Alcohols: Their Distribution in Raw Soybean Tissues and Formation in Crude Soy Protein Concentrate by Lipoxygenase. Agric. Biol. Chem. 1970, 34, 1420–1423. [Google Scholar] [CrossRef]
- Concepcion, J.C.T.; Ouk, S.; Riedel, A.; Calingacion, M.; Zhao, D.; Ouk, M.; Garson, M.J.; Fitzgerald, M.A. Quality Evaluation, Fatty Acid Analysis and Untargeted Profiling of Volatiles in Cambodian Rice. Food Chem. 2018, 240, 1014–1021. [Google Scholar] [CrossRef]
- Snyder, J.; Frankel, E.; Selke, E.; Warner, K. Comparison of Gas Chromatographic Methods for Volatile Lipid Oxidation Compounds in Soybean Oil. J. Am. Oil Chem. Soc. 1988, 65, 1617–1620. [Google Scholar] [CrossRef]
- Climate-Data. Available online: http://en.climate-data.org (accessed on 15 October 2019).
- Zhang, Y.; Guo, S.; Liu, Z.; Chang, S.K. Off-Flavor Related Volatiles in Soymilk as Affected by Soybean Variety, Grinding, and Heat-Processing Methods. J. Agric. Food Chem. 2012, 60, 7457–7462. [Google Scholar] [CrossRef]
- Yaylayan, V. Precursors, Formation and Determination of Furan in Food. J. Verbrauch. Lebensm. 2006, 1, 5–9. [Google Scholar] [CrossRef]
- Min, D.; Callison, A.; Lee, H. Singlet Oxygen Oxidation for 2-pentylfuran and 2-pentenyfuran Formation in Soybean Oil. J. Food Sci. 2003, 68, 1175–1178. [Google Scholar] [CrossRef]
- Whitfield, F.B.; Mottram, D.S. Volatiles from Interactions of Maillard Reactions and Lipids. Crit. Rev. Food Sci. Nutr. 1992, 31, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Leffingwell, J.C.; Alford, E.; Leffingwell, D. Identification of the Volatile Constituents of Raw Pumpkin (Cucurbita Pepo, L.) by Dynamic Headspace Analyses. Leffingwell Rep. 2015, 7, 1–14. [Google Scholar]
- Cheesbrough, T.M. Changes in the Enzymes for Fatty Acid Synthesis and Desaturation during Acclimation of Developing Soybean Seeds to Altered Growth Temperature. Plant Physiol. 1989, 90, 760–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulen, H.; Eris, A. Effect of Heat Stress on Peroxidase Activity and Total Protein Content in Strawberry Plants. Plant Sci. 2004, 166, 739–744. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, A.; Tindwani, C.; Jain, M. Lipoxygenase Isozymes and Trypsin Inhibitor Activities in Soybean as Influenced by Growing Location. Food Chem. 2003, 83, 79–83. [Google Scholar] [CrossRef]
- Yuan, J.S.; Himanen, S.J.; Holopainen, J.K.; Chen, F.; Stewart, C.N., Jr. Smelling Global Climate Change: Mitigation of Function for Plant Volatile Organic Compounds. Trends Ecol. Evol. 2009, 24, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Croteau, R.; Davis, E.; Hartmann, T.; Hemscheidt, T.; Sanz-Cervera, J.; Shen, B.; Stocking, E.; Williams, R. Biosynthesis: Aromatic Polyketides, Isoprenoids, Alkaloids; Springer: Berlin, Germany, 2003; pp. 54–91. [Google Scholar]
- Cho, I.H.; Lee, H.J.; Kim, Y. Differences in the Volatile Compositions of Ginseng Species (Panax sp.). J. Agric. Food Chem. 2012, 60, 7616–7622. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the Aroma of Grapes and Wines: A Review. S. Afr. J. Enol. Vitic. 1983, 4, 49–58. [Google Scholar] [CrossRef]
- Olivier, J.G.; Schure, K.; Peters, J. Trends in Global CO2 and Total Greenhouse Gas Emissions; PBL Netherlands Environmental Assessment Agency: Hague, The Netherlands, 2017. [Google Scholar]
- Wishart, D.S. Metabolomics: Applications to Food Science and Nutrition Research. Trends Food Sci. Technol. 2008, 19, 482–493. [Google Scholar] [CrossRef]
- Shi, A.; Chen, P.; Zhang, B.; Hou, A. Genetic Diversity and Association Analysis of Protein and Oil Content in food-grade Soybeans from Asia and the United States. Plant Breed 2010, 129, 250–256. [Google Scholar] [CrossRef]
- Maestri, D.M.; Labuckas, D.O.; Meriles, J.M.; Lamarque, A.L.; Zygadlo, J.A.; Guzmán, C.A. Seed Composition of Soybean Cultivars Evaluated in Different Environmental Regions. J. Sci. Food Agric. 1998, 77, 494–498. [Google Scholar] [CrossRef]
- Dornbos, D.; Mullen, R. Soybean Seed Protein and Oil Contents and Fatty Acid Composition Adjustments by Drought and Temperature. J. Am. Oil Chem. Soc. 1992, 69, 228–231. [Google Scholar] [CrossRef]
- MacLeod, G.; Ames, J.; Betz, N.L. Soy Flavor and Its Improvement. Crit. Rev. Food Sci. Nutr. 1988, 27, 219–400. [Google Scholar] [CrossRef] [PubMed]
- Medic, J.; Atkinson, C.; Hurburgh, C.R. Current Knowledge in Soybean Composition. J. Am. Oil Chem. Soc. 2014, 91, 363–384. [Google Scholar] [CrossRef]
- Oberg, J.; Spenger, C.; Wang, F.; Andersson, A.; Westman, E.; Skoglund, P.; Sunnemark, D.; Norinder, U.; Klason, T.; Wahlund, L. Age Related Changes in Brain Metabolites Observed by 1H MRS in APP/PS1 Mice. Neurobiol. Aging 2008, 29, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA) for Multivariate Association between Bioactive Compounds and Functional Properties in Foods: A Critical Perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Wills, R.; McGlasson, W. Effect of Storage Temperature on Apple Volatiles Associated with Low Temperature Breakdown. J. Hortic. Sci. 1971, 46, 115–120. [Google Scholar] [CrossRef]
- Vallat, A.; Gu, H.; Dorn, S. How Rainfall, Relative Humidity and Temperature Influence Volatile Emissions from Apple Trees In Situ. Phytochemistry 2005, 66, 1540–1550. [Google Scholar] [CrossRef]
- The Institute for Genomic Research (TIGR). Available online: http://mev.tm4.org (accessed on 10 October 2019).
Sample Availability: Not available. |




| Province | Latitude | Longitude | Annual Mean Temperature (°C) | Annual Mean Precipitation (mm) |
|---|---|---|---|---|
| Korea | ||||
| Gyeongi | 11° 7′ 15.744″ N | 105° 32′ 0.5748″ E | 11.7 | 1240 |
| Gangwon | 37° 52′ 52.7268″ N | 37° 52′ 52.7268″ N | 10.9 | 1307 |
| Chungcheongbuk | 36° 56′ 10.068″ N | 127° 41′ 44.736″ E | 10.8 | 1239 |
| Chungcheongnam | 36° 48′ 33.5196″ N | 127° 9′ 36.1512″ E | 11.8 | 1229 |
| Jeollabuk | 35° 47′ 52.8432″ N | 126° 53′ 31.9632″ E | 12.8 | 1251 |
| Jeollnam | 35° 1′ 37.308″ N | 126° 43′ 15.024″ E | 13.9 | 1264 |
| Kyeongsangbuk | 35° 59′ 18.312″ N | 128° 56′ 31.2″ E | 12.6 | 1026 |
| Kyeongsangnam | 35° 31′ 48.792″ N | 128° 30′ 28.116″ E | 13.3 | 1248 |
| North America | ||||
| Illinois | 40° 37′ 59.25″ N | 89° 23′ 54.7044″ W | 11.42 | 947.93 |
| Indiana | 40° 16′ 1.8948″ N | 86° 8′ 5.6508″ W | 11.1 | 1011 |
| Minnesota | 46° 43′ 46.3908″ N | 94° 41′ 9.2328″ W | 7.3 | 807 |
| Michigan | 44° 18′ 53.4312″ N | 85° 36′ 8.5104″ W | 8.6 | 890 |
| Quebec | 52° 56′ 23.694″ N | 73° 32′ 56.8788″ W | 4.8 | 1001 |
| Ontario | 51° 15′ 13.5972″ N | 85° 19′ 23.5632″ W | 7.1 | 775.9 |
| China | ||||
| Heilongjian | 45° 37′ 17.9832″ N | 126° 14′ 35.3466″ E | 3.4 | 562 |
| Jilin | 42° 31′ 40.44″ N | 125° 40′ 40.7994″ E | 4.9 | 784 |
| Liaoning | 40° 1′ 44.1114″ N | 124° 17′ 4.4484″ E | 9.0 | 1040 |
| Hebei | 38° 16′ 53.5578″ N | 114° 41′ 29.7276″ E | 13.2 | 517 |
| Shandong | 41° 1′ 59.0874″ N | 113° 6′ 25.6314″ E | 14.1 | 676 |
| Hubei | 30° 13′ 35.3634″ N | 115° 3′ 49.4634″ E | 17.0 | 1396 |
| Anhui | 33° 57′ 22.248″ N | 116° 47′ 20.5434″ E | 15.2 | 728 |
| Zhejiang | 30° 42′ 1.8″ N | 121° 0′ 37.3314″ E | 16.2 | 1118 |
| Fujian | 25° 6′ 50.796″ N | 99° 9′ 44.28″ E | 20.7 | 1677 |
| Jiangxi | 31° 21′ 54.6474″ N | 118° 23′ 22.8114″ E | 17.2 | 1475 |
| Guangdong | 24° 48′ 4.068″ N | 113° 35′ 33.7554″ E | 21.0 | 1499 |
| Retention Index (RI) Cal 1 | RI Ref 2 | Volatile Compounds | VIP Values | Identification (ID) 3 | |
|---|---|---|---|---|---|
| Negative direction | |||||
| 1289 | 1288 | Heptan-4-ol | 2.29 | B | |
| 1151 | Butan-1-ol | 2.19 | A | ||
| 1217 | Butyl butanoate | 2.00 | A | ||
| 1285 | 1287 | Octanal | 1.97 | B | |
| 1175 | Butyl prop-2-enoate | 1.89 | A | ||
| 1642 | 1631 | 5-Methyl-2-propan-2-ylcyclohexan-1-ol | 1.88 | B | |
| 1067 | Butyl acetate | 1.88 | A | ||
| 1141 | Butyl propanoate | 1.84 | A | ||
| 1391 | Nonanal | 1.83 | A | ||
| 1027 | Toluene | 1.80 | A | ||
| 1181 | Heptanal | 1.75 | A | ||
| 1122 | Heptan-4-one | 1.74 | A | ||
| 1688 | 1694 | 5-Ethyloxolan-2-one | 1.67 | B | |
| 1273 | 1,2,3-Trimethylbenzene | 1.63 | A | ||
| 1178 | Heptan-2-one | 1.55 | A | ||
| 995 | Acetonitrile | 1.54 | A | ||
| 1210 | (E)-Hex-2-enal | 1.54 | A | ||
| 1234 | Ethyl hexanoate | 1.49 | A | ||
| 1317 | (E)-Hept-2-enal | 1.45 | A | ||
| 1190 | Limonene | 1.40 | A | ||
| 959 | 968 | 1-Butoxybutane | 1.37 | B | |
| 1230 | 2-Pentylfuran | 1.30 | A | ||
| 605 | Acetaldehyde | 1.27 | A | ||
| 1162 | Myrcene | 1.27 | A | ||
| 1279 | 3-Hydroxybutan-2-one | 1.22 | A | ||
| Positive direction | |||||
| 1493 | 2-Ethylhexan-1-ol | 1.75 | A | ||
| RI Cal 1 | RI Ref 2 | Volatile Compounds | VIP Values | ID 3 |
|---|---|---|---|---|
| Negative direction | ||||
| 1493 | 2-Ethylhexan-1-ol | 2.73 | A | |
| 1194 | 2,5-Dimethylhexan-2-ol | 2.29 | C | |
| 1250 | Styrene | 2.16 | A | |
| 850 | 2-Methylfuran | 2.02 | A | |
| <600 | 2-Methylprop-1-ene | 1.93 | C | |
| 792 | Propan-2-one | 1.88 | A | |
| 861 | 2-Methylprop-2-enal | 1.85 | A | |
| 600 | Hexane | 1.80 | A | |
| 810 | Methyl acetate | 1.48 | A | |
| 1304 | 1312 | 2-Methylpentan-1-ol | 1.40 | B |
| 1285 | 1287 | Octanal | 1.37 | B |
| 1175 | Butyl prop-2-enonate | 1.29 | A | |
| 1129 | 1-Methoxypropan-2-ol | 1.23 | A | |
| 1181 | Heptanal | 1.22 | A | |
| 1027 | Toluene | 1.21 | A | |
| Positive direction | ||||
| 1449 | Oct-1-en-3-ol | 2.32 | A | |
| 900 | 900 | Nonane | 2.11 | B |
| 1598 | 4-Methyloxolan-2-one | 1.67 | C | |
| 1289 | 1288 | Heptan-4-ol | 1.54 | B |
| 1151 | Butan-1-ol | 1.48 | A | |
| 1252 | Octan-3-one | 1.43 | A | |
| 1220 | Butyl butanoate | 1.35 | A | |
| 1850 | 3-Hydroxy-2,4,4-trimethylpentyl 2-methylpropanoate | 1.33 | C | |
| 1642 | 1631 | 5-Methyl-2-propan-2-ylcyclohexan-1-ol | 1.26 | B |
| 1067 | Butyl acetate | 1.26 | A | |
| 1140 | Butyl propanoate | 1.24 | A | |
| 1391 | Nonanal | 1.23 | A | |
| Nation | Province | Location | Labeling 1 |
|---|---|---|---|
| Korea | Gyeonggi | Anseong | GGIC |
| Icheon | GGAS | ||
| Gangwon | Chuncheon | GWCC | |
| Yeongwol | GWYW | ||
| Chungcheongbuk | Eumseong | CBES | |
| Chungcheongnam | Cheonan | CNCA | |
| Gongju | CNGJ | ||
| Jeollabuk | Gimje | JBGJ | |
| Imsil | JBIS | ||
| Jeollanam | Naju | JNNJ | |
| Yeonggwang | JNYG | ||
| Kyeongsangbuk | Cheongdo | KBCD | |
| Uiseong | KBES | ||
| Yeongcheon | KBYC | ||
| Kyeongsangnam | Changnyeong | KNCN | |
| Miryang | KNMY | ||
| Geochang | KNGC | ||
| China | Neimenggu | Ulanhot | INUL |
| Heilongjiang | Harbin | HEHA | |
| Jilin | Meihekou | JIME | |
| Liaoning | Dandong | LIDA | |
| Hebei | Shijiazhuang | HESH | |
| Shandong | Jining | SHJI | |
| Anhui | Huaibei | ANHU | |
| Hubei | Huangshi | HUHU | |
| Zhejiang | Pinghu | ZHPI | |
| Jiangxi | Jiujiang | JIJI | |
| Fujian | Longyan | FULO | |
| Guangdong | Shaoguan | GUSH | |
| Guangxi | Hechi | GUBA | |
| The United States (North America) | Illinois | IL | |
| Indiana | IN | ||
| Minnesota | MN | ||
| Michigan | MI | ||
| Canada (North America) | Quebec | QB | |
| Ontario | ON |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Kim, S.Y.; Lee, S.M.; Lee, D.Y.; Shin, B.K.; Kang, D.J.; Choi, H.-K.; Kim, Y.-S. Discrimination of Cultivated Regions of Soybeans (Glycine max) Based on Multivariate Data Analysis of Volatile Metabolite Profiles. Molecules 2020, 25, 763. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25030763
Kim S-Y, Kim SY, Lee SM, Lee DY, Shin BK, Kang DJ, Choi H-K, Kim Y-S. Discrimination of Cultivated Regions of Soybeans (Glycine max) Based on Multivariate Data Analysis of Volatile Metabolite Profiles. Molecules. 2020; 25(3):763. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25030763
Chicago/Turabian StyleKim, So-Yeon, So Young Kim, Sang Mi Lee, Do Yup Lee, Byeung Kon Shin, Dong Jin Kang, Hyung-Kyoon Choi, and Young-Suk Kim. 2020. "Discrimination of Cultivated Regions of Soybeans (Glycine max) Based on Multivariate Data Analysis of Volatile Metabolite Profiles" Molecules 25, no. 3: 763. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25030763