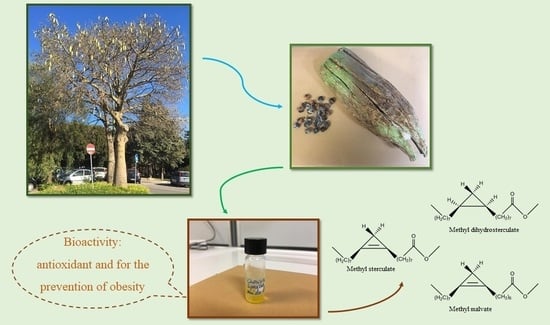
Ceiba speciosa (A. St.-Hil.) Seeds Oil: Fatty Acids Profiling by GC-MS and NMR and Bioactivity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Profiling
2.2. Antioxidant Activity
2.3. Inhibition of Enzymes Linked to Obesity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Extraction Procedure
3.4. Gas Chromatography—Mass Spectrometry (GC-MS) Analyses
3.5. Nuclear Magnetic Resonance (NMR) Analyses
3.6. Radical Scavenging Activity Assays
3.7. β-Carotene Bleaching Test
3.8. Ferric Reducing Ability Power (FRAP) Assay
3.9. Pancreatic Lipase Inhibitory Activity
3.10. Carbohydrates-Hydrolysing Enzymes Inhibitory Activity
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Rabe, K.; Lehrke, M.; Parhofer, K.G.; Broedl, U.C. Adipokines and insulin resistance. Mol. Med. 2008, 14, 741–751. [Google Scholar] [CrossRef]
- Ruscica, M.; Baragetti, A.; Catapano, A.L.; Norata, G.D. Translating the biology of adipokines in atherosclerosis and cardiovascular diseases: Gaps and open questions. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 379–395. [Google Scholar] [CrossRef]
- American Diabetes Association. 8. Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. 1), S81–S89. [CrossRef] [PubMed] [Green Version]
- Găman, M.; Epingeac, M.; Diaconu, C.; Găman, A. Oxidative stress levels are increased in type 2 diabetes mellitus and obesity. J. Hypertens. 2019, 37, e265. [Google Scholar] [CrossRef]
- Luczaj, L.; Pieroni, A.; Tardio, J.; Pardo-de-Santayana, M.; Soukand, R.; Svanberg, I.; Kalle, R. Wild food plant use in 21st century Europe: The disappearance of old traditions and the search for new cuisines involving wild edibles. Acta Soc. Bot. Pol. 2012, 81, 359–370. [Google Scholar] [CrossRef]
- Marrelli, M.; Loizzo, M.R.; Nicoletti, M.; Menichini, F.; Conforti, F. In vitro investigation of the potential health benefits of wild Mediterranean dietary plants as antiobesity agents with α-amylase and pancreatic lipase inhibitory activities. J. Sci. Food Agric. 2014, 94, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Joly, A.B. Botany: An Introduction to Plant Taxonomy, 10th ed.; Säo Paulo: National Publishing Company: Rio de Janeiro, Brazil, 1991; p. 462. [Google Scholar]
- Bhattacharyya, B. Golden Greens: The Amazing World of Plants; The Energy and Resources Institute (TERI): New Delhi, India, 2016; pp. 59–61. [Google Scholar]
- Pasta, S.; La Mantia, T.; Badalamenti, E. A casual alien plant new to Mediterranean Europe: Ceiba speciosa (Malvaceae) in the suburban area of Palermo (NW Sicily, Italy). Anales. Jard. Bot. Madrid 2014, 71, e010. [Google Scholar] [CrossRef] [Green Version]
- Lojacono-Pojero, M. Sulla Chorisia speciosa S. Hil. a Palermo. Bollettino della Società Orticola del Mutuo Soccorso 1911, 9, 13–15. [Google Scholar]
- Bruno, F. La Chorisia speciosa St. Hill. e la sua coltivazione in Sicilia. Lavori dell’Istituto Botanico e del Giardino Coloniale di Palermo 1952, 13, 1–19. [Google Scholar]
- Refaat, J.; Yehia, S.; Ramadan, M.; Kamel, M.; Han, J.; Isoda, H. Comparative polyphenol contents, DPPH radical scavenging properties and effects on adipogenesis of Chorisia chodatii and Chorisia speciosa. J. Herb. Drugs 2015, 5, 193–207. [Google Scholar]
- Hafez, S.S.; Abdel-Ghani, A.E.; El-Shazly, A.M. Pharmacognostical and antibacterial studies of Chorisia speciosa St. Hill. flower (Bombacaeae). Mans. J. Pharm. Sci. 2003, 19, 40–43. [Google Scholar]
- Coussio, J.D. Isolation of rhoifolin from Chorisia species (Bombacaceae). Experientia 1964, 20, 562. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Ali, M.; Rais, I.; Mir, S.R. Isolation of apigenin derivatives from the leaves of Chorisia speciosa, Cordia dichotoma, Mentha piperita and roots of Pluchea lanceolata. Trop. J. Nat. Prod. Res. 2017, 1, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Scogin, R. Reproductive phytochemistry of Bombacaceae: Floral anthocyanins and nectar constituents. Aliso 1986, 11, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.A. Phytochemical and Biological Investigation of Certain Plants Containing Pigments. Ph.D. Thesis, Mansoura University, Mansoura, Egypt, 2009. [Google Scholar]
- Petronici, C.; Bazan, E.; Panno, M.; Averna, V. Composizione acidica e struttura gliceridica dell’olio dei semi di Chorisia speciosa St. Hil. Riv. Ital. Sostanze Gr. 1974, 51, 428–431. [Google Scholar]
- Beleski-Carneiro, E.B.; Ganter, J.L.; Reicher, F. Structural aspects of the exudate from the fruit of Chorisia speciosa St. Hil. Int. J. Biol. Macromol. 1999, 26, 219–224. [Google Scholar] [CrossRef]
- Di Fabio, J.L.; Dutton, G.G.; Moyna, P. The structure of Chorisia speciosa gum. Carbohydr. Res. 1982, 99, 41–50. [Google Scholar] [CrossRef]
- Beleski-Carneiro, E.; Sugui, J.; Reicher, F. Structural and biological features of a hydrogel from seed coats of Chorisia speciosa. Phytochemistry 2002, 61, 157–163. [Google Scholar] [CrossRef]
- Beleski-Carneiro, E.; Sierakowski, M.R.; Ganter, J.L.; Zawadzki-Baggio, S.F.; Reichera, F. Polysaccharides from Chorisia speciosa St. Hil. Prog. Biotechnol. 1996, 14, 549–559. [Google Scholar] [CrossRef]
- Caffini, N.O.; Lufrano, N.S. Mucílagos de Malvales. I-Análisis fitoquímico del mucílago de hojas de Chorisia speciosa St.Hil. (Bombacaceae). Rev. Farm. 1978, 120, 75–80. [Google Scholar]
- Nasr, E.M.; Assaf, M.H.; Darwish, F.M.; Ramadan, M.A. Phytochemical and biological study of Chorisia speciosa A. St. Hil. cultivated in Egypt. J. Pharmacogn. Pharmacol. 2018, 7, 649–656. [Google Scholar]
- Refaat Fahim, J.; Hegazi, G.A.; Abo El-Fadl, R.E. Abd Al-Magid, M.R.; Desoukey, S.Y.; Ramadan, M.A.; Kamel, M.S. Production of rhoifolin and tiliroside from callus cultures of Chorisia chodatii and Chorisia speciosa. Phytochem. Lett. 2015, 13, 218–227. [Google Scholar] [CrossRef]
- Khan, A.; Asadsaeed, M.; Chaudhary, M.A.; Ahmad, Q.; Ansari, F. Antimicrobial, anti-inflammatory and antipyretic activity of Chorisia speciosa leaves. (Bombacaceae). Int. J. Biol. Pharm. Allied Sci. 2015, 4, 6826–6838. [Google Scholar]
- Bohannon, M.B.; Kleiman, R. Cyclopropene fatty acids of selected seed oils from Bombacaceae, Malvaceae, and Sterculiaceae. Lipids 1978, 13, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Aued-Pimentel, S.; Lago, J.H.; Chaves, M.H.; Kumagai, E.E. Evaluation of a methylation procedure to determine cyclopropenoids fatty acids from Sterculia striata St. Hil. Et Nauds seed oil. J. Chromatogr. A 2004, 1054, 235–239. [Google Scholar] [CrossRef]
- Berry, S.K. The characteristics of the kapok (Ceiba pentadra Gaertn.) seed oil. Pertanika J. Trop. Agric. Sci. 1979, 21, 1–4. [Google Scholar]
- Kiran Ravi, C.; Rao, T. Lipid Profiling by GC-MS and Anti-inflammatory Activities of Ceiba pentandra Seed Oil. J. Biol. Act. Prod. Nat. 2014, 4, 62–70. [Google Scholar] [CrossRef]
- Knothe, G. NMR Characterization of Dihydrosterculic Acid and its Methyl Ester. Lipids 2006, 41, 393–396. [Google Scholar] [CrossRef]
- Hernando, J.; Matía, M.P.; Novella, J.L.; Alvarez-Builla, J. Synthesis of sterculic acid. Arkivoc 2002, 2002, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Howarth, O.W.; Vlahov, G. 13C Nuclear magnetic resonance study of cyclopropenoid triacylglycerols. Chem. Phys. Lipids 1996, 81, 81–85. [Google Scholar] [CrossRef]
- Dörr, J.A.; Bitencourt, S.; Bortoluzzi, L.; Alves, C.; Silva, J.; Stoll, S.; Pinteus, S.; Boligon, A.A.; Santos, R.C.V.; Laufer, S.; et al. In vitro activities of Ceiba speciosa (A.St.-Hil) Ravenna aqueous stem bark extract. Nat. Prod. Res. 2019, 33, 3441–3444. [Google Scholar] [CrossRef]
- Krüger Cardoso Malheiros, C.; Sayonara Barbosa Silva, J.; Hofmann, T.C.; Martins Messina, T.; Manfredini, V.; da Costa Escobar Piccoli, J.; Faoro, D.; Souza Oliveira, L.F.; Mansur Machado, M.; Moreira Farias, F. Preliminary in vitro assessment of the potential toxicity and antioxidant activity of Ceiba speciosa (A. St.-Hill) Ravenna (Paineira). Braz. J. Pharm. 2017, 53, e16098. [Google Scholar] [CrossRef] [Green Version]
- El-Alfy, T.S.; El-Sawi, S.A.; Sleem, A.; Moawad, D.M. Investigation of flavonoidal content and biological activities of Chorisia insignis Hbk. leaves. Aust. J. Basic Appl. Sci. 2010, 4, 1334–1348. [Google Scholar]
- Patil, A.; Thakurdesai, P.A.; Pawar, S.; Soni, K. Evaluation of ethanolic leaf extract of Ceiba pentandra for anti-obesity and hypolipidaemic activity in cafeteria diet (cd) treated wistar albino rats. Int. J. Pharm. Sci. 2012, 3, 2664–2668. [Google Scholar] [CrossRef]
- Giardinieri, A.; Schicchi, R.; Geraci, A.; Rosselli, S.; Maggi, F.; Fiorinie, D.; Ricciutellif, M.; Loizzo, M.R.; Bruno, M.; Pacettia, D. Fixed oil from seeds of narrow-leaved ash (F. angustifolia subsp. angustifolia): Chemical profile, antioxidant and antiproliferative activities. Food Res. Int. 2019, 119, 369–377. [Google Scholar] [CrossRef]
- Ichihara, K.; Shibahara, A.; Yamamoto, K.; Nakayama, T. An improved method for rapid analysis of the fatty acids of glycerolipids. Lipids 1996, 31, 535–539. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Sut, S.; Dall’Acqua, S.; Ilardi, V.; Leporini, M.; Falco, T.; Sicari, V.; Bruno, M. High-Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry (HPLC-ESI-MSn) Analysis and Bioactivity Useful for Prevention of “Diabesity” of Allium commutatum Guss. Plant Foods Hum. Nutr. 2019, in press. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Lucci, P.; Núñez, O.; Tundis, R.; Balzano, M.; Frega, N.G.; Conte, L.; Moret, S.; Filatova, D.; Moyano, E.; et al. Native colombian fruits and their by-products: Phenolic profile, antioxidant activity and hypoglycaemic potential. Foods 2019, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Loizzo, M.R.; Sicari, V.; Pellicanò, T.; Xiao, J.; Poiana, M.; Tundis, R. Comparative analysis of chemical composition, antioxidant and anti-proliferative activities of Italian Vitis vinifera by-products for a sustainable agro-industry. Food Chem. Toxicol. 2019, 127, 127–134. [Google Scholar] [CrossRef]
- El-shiekh, R.A.; Al-Mahdy, D.A.; Hifnawy, M.S.; Abdel-Sattar, E.A. In-vitro screening of selected traditional medicinal plants for their anti-obesity and anti-oxidant activities. South Afr. J. Bot. 2019, 123, 43–50. [Google Scholar] [CrossRef]
Sample Availability: Samples of the oil are available from the authors. |




| Peak | Rt (min) | Fatty acid a | % |
|---|---|---|---|
| 1 | 15.78 | Myristic acid | 0.38 ± 0.01 |
| 2 | 17.16 | Palmitoleic acid | 1.42 ± 0.24 |
| 3 | 17.33 | Palmitic acid | 19.56 ± 1.32 |
| 4 | 17.81 | Margaric acid | 1.41 ± 0.02 |
| 5 | 18.37 | Malvalic acid b | 16.15 ± 2.14 |
| 6 | 18.54 | Linoleic acid | 28.22 ± 2.56 |
| 7 | 18.63 | Oleic acid | 8.93 ± 0.21 |
| 8 | 18.71 | Stearic acid | 4.33 ± 0.14 |
| 9 | 19.03 | Sterculic acid b | 11.11 ± 2.88 |
| 10 | 19.26 | Dihydrosterculic acid b | 2.74 ± 0.16 |
| 11 | 19.81 | Gondoic acid | 0.40 ± 0.03 |
| 12 | 19.95 | Arachidic acid | 1.27 ± 0.13 |
| 13 | 21.14 | Behenic acid | 1.35 ± 0.21 |
| 14 | 22.58 | Lignoceric acid | 0.52 ± 0.03 |
| Total identified | 97.79 |
| DPPH IC50 (µg/mL) | ABTS IC50 (µg/mL) | FRAP μMFe (II)/g | β-carotene Bleaching Test t = 30 Min % at 100 μg/mL | β-carotene Bleaching Test t = 60 Min % at 100 μg/mL | |
|---|---|---|---|---|---|
| Chorisia speciosa | 77.44 ± 2.93 **** | 10.21 ± 2.35 ** | 21.12 ± 2.14 **** | 37.36 ± 2.05 **** | 36.98 ± 2.11 **** |
| Positive control | |||||
| Ascorbic acid | 5.04 ± 0.84 | 1.76 ± 0.06 | |||
| BHT | 63.20 ± 2.34 | ||||
| Propyl gallate | 0.09 ± 0.04 | 0.09 ± 0.04 |
| Sample | α-Amylase | α-Glucosidase | Lipase |
|---|---|---|---|
| C. speciosa | 158.22 ± 2.89 **** | 135.69 ± 2.68 **** | 127.57 ± 2.98 **** |
| Positive control | |||
| Acarbose | 50.01 ± 0.92 | 35.52 ± 1.23 | |
| Orlistat | 37.63 ± 1.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosselli, S.; Tundis, R.; Bruno, M.; Leporini, M.; Falco, T.; Gagliano Candela, R.; Badalamenti, N.; Loizzo, M.R. Ceiba speciosa (A. St.-Hil.) Seeds Oil: Fatty Acids Profiling by GC-MS and NMR and Bioactivity. Molecules 2020, 25, 1037. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25051037
Rosselli S, Tundis R, Bruno M, Leporini M, Falco T, Gagliano Candela R, Badalamenti N, Loizzo MR. Ceiba speciosa (A. St.-Hil.) Seeds Oil: Fatty Acids Profiling by GC-MS and NMR and Bioactivity. Molecules. 2020; 25(5):1037. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25051037
Chicago/Turabian StyleRosselli, Sergio, Rosa Tundis, Maurizio Bruno, Mariarosaria Leporini, Tiziana Falco, Rossella Gagliano Candela, Natale Badalamenti, and Monica R. Loizzo. 2020. "Ceiba speciosa (A. St.-Hil.) Seeds Oil: Fatty Acids Profiling by GC-MS and NMR and Bioactivity" Molecules 25, no. 5: 1037. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25051037