Flow-Based Dynamic Approach to Assess Bioaccessible Zinc in Dry Dog Food Samples
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Chamber Configuration
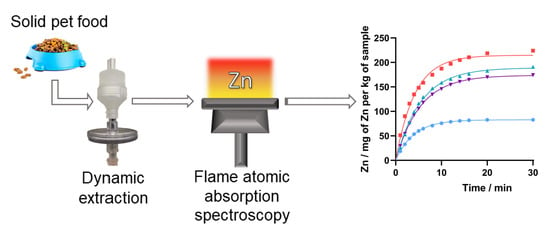
2.2. Study of the Extraction Procedure
2.3. Zinc Bioaccessibility Assessment
3. Materials and Methods
3.1. Chemicals and Solutions
3.2. Samples
3.3. Flow-Based Dynamic Extraction Apparatus
3.4. Extraction Chamber
3.5. Flow-Based Dynamic Extraction Procedure
3.6. Static Batch Extraction Procedure
3.7. Determination of Zinc and Leaching Profile
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Case, L.P.; Carey, D.P.; Hirakawa, D.A.; Daristotle, L. Canine and Feline Nutrition: A Resource for Companion Animal Professionals; Mosby Inc.: St Louis, MO, USA, 2000; pp. 41–42. [Google Scholar]
- Cummings, J.E.; Kovacic, J.P. The ubiquitous role of zinc in health and disease. J. Vet. Emerg. Crit. Care 2009, 19, 215–240. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; Pinto, E.; Matos, E.; Castanheira, F.; Almeida, A.A.; Baptista, C.S.; Segundo, M.A.; Fonseca, A.J.M.; Cabrita, A.R.J. Mineral composition of dry dog foods: Impact on nutrition and potential toxicity. J. Agric. Food Chem. 2018, 66, 7822–7830. [Google Scholar] [CrossRef] [PubMed]
- Perring, L.; Nicolas, M.; Andrey, D.; Rime, C.F.; Richoz-Payot, J.; Dubascoux, S.; Poitevin, E. Development and validation of an ED-XRF method for the fast quantification of mineral elements in dry pet food samples. Food Anal. Meth. 2017, 10, 1469–1478. [Google Scholar] [CrossRef]
- FEDIAF. The European Pet Food Industry Federation, Nutritional Guidelines For Complete and Complementary Pet Food for Cats and Dogs; FEDIAF: Bruxelles, Belgium, 2019. [Google Scholar]
- European Comission. Commission Implementing Regulation (EU) 2016/1095 of 6 July 2016 concerning the authorisation of Zinc acetate dihydrate, Zinc chloride anhydrous, Zinc oxide, Zinc sulphate heptahydrate, Zinc sulphate monohydrate, Zinc chelate of amino acids hydrate, Zinc chelate of protein hydrolysates, Zinc chelate of glycine hydrate (solid) and Zinc chelate of glycine hydrate (liquid) as feed additives for all animal species and amending Regulations (EC) No 1334/2003, (EC) No 479/2006, (EU) No 335/2010 and Implementing Regulations (EU) No 991/2012 and (EU) No 636/2013. Available online: https://eur-lex.europa.eu/eli/reg_impl/2016/1095/oj (accessed on 1 March 2020).
- Gabaza, M.; Shumoy, H.; Muchuweti, M.; Vandamme, P.; Raes, K. Baobab fruit pulp and mopane worm as potential functional ingredients to improve the iron and zinc content and bioaccessibility of fermented cereals. Innov. Food Sci. Emerg. Technol. 2018, 47, 390–398. [Google Scholar] [CrossRef]
- Ramírez-Ojeda, A.M.; Moreno-Rojas, R.; Sevillano-Morales, J.; Cámara-Martos, F. Influence of dietary components on minerals and trace elements bioaccessible fraction in organic weaning food: A probabilistic assessment. Eur. Food Res. Technol. 2016, 243, 639–650. [Google Scholar] [CrossRef]
- Leufroy, A.; Noel, L.; Beauchemin, D.; Guerin, T. Bioaccessibility of total arsenic and arsenic species in seafood as determined by a continuous online leaching method. Anal. Bioanal. Chem. 2012, 402, 2849–2859. [Google Scholar] [CrossRef]
- Intawongse, M.; Dean, J.R. In-vitro testing for assessing oral bioaccessibility of trace metals in soil and food samples. Trac-Trends Anal. Chem. 2006, 25, 876–886. [Google Scholar] [CrossRef]
- Gabaza, M.; Shumoy, H.; Louwagie, L.; Muchuweti, M.; Vandamme, P.; Du Laing, G.; Raes, K. Traditional fermentation and cooking of finger millet: Implications on mineral binders and subsequent bioaccessibility. J. Food Compos. Anal. 2018, 68, 87–94. [Google Scholar] [CrossRef]
- Iturbide-Casas, M.A.; Molina-Recio, G.; Camara-Martos, F. Manganese preconcentration and speciation in bioaccessible fraction of enteral nutrition formulas by cloud point extraction (CPE) and atomic absorption spectroscopy. Food Anal. Meth. 2018, 11, 2758–2766. [Google Scholar] [CrossRef]
- Cardoso, C.; Afonso, C.; Lourenço, H.; Costa, S.; Nunes, M.L. Bioaccessibility assessment methodologies and their consequences for the risk–benefit evaluation of food. Trends Food Sci. Technol. 2015, 41, 5–23. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A.; Romarís-Hortas, V.; Moscoso-Pérez, C.; López-Mahía, P.; Muniategui-Lorenzo, S.; Bermejo-Barrera, P.; Prada-Rodríguez, D. In-vivo and in-vitro testing to assess the bioaccessibility and the bioavailability of arsenic, selenium and mercury species in food samples. Trac-Trends Anal. Chem. 2011, 30, 324–345. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food - an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etcheverry, P.; Grusak, M.A.; Fleige, L.E. Application of in vitro bioaccessibility and bioavailability methods for calcium, carotenoids, folate, iron, magnesium, polyphenols, zinc, and vitamins B(6), B(12), D, and E. Front. Physiol. 2012, 3, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas-González, R.; Viuda-Martos, M.; Pérez-Alvarez, J.A.; Fernández-López, J. In vitro digestion models suitable for foods: Opportunities for new fields of application and challenges. Food Res. Int. 2018, 107, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; Le Feunteun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models? Crit. Rev. Food Sci. Nutr. 2018, 58, 2239–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Zelst, M.; Hesta, M.; Alexander, L.G.; Gray, K.; Bosch, G.; Hendriks, W.H.; Du Laing, G.; De Meulenaer, B.; Goethals, K.; Janssens, G.P.J. In vitro selenium accessibility in pet foods is affected by diet composition and type. Br. J. Nutr. 2015, 113, 1888–1894. [Google Scholar] [CrossRef] [Green Version]
- Santos, W.P.C.; Ribeiro, N.M.; Santos, D.; Korn, M.G.A.; Lopes, M.V. Bioaccessibility assessment of toxic and essential elements in produced pulses, Bahia, Brazil. Food Chem. 2018, 240, 112–122. [Google Scholar] [CrossRef]
- Devaraju, S.K.; Thatte, P.; Prakash, J.; Lakshmi, J.A. Bioaccessible iron and zinc in native and fortified enzyme hydrolyzed casein and soya protein matrices. Food Biotechnol. 2016, 30, 233–248. [Google Scholar] [CrossRef]
- Theodoropoulos, V.C.T.; Turatti, M.A.; Greiner, R.; Macedo, G.A.; Pallone, J.A.L. Effect of enzymatic treatment on phytate content and mineral bioacessability in soy drink. Food Res. Int. 2018, 108, 68–73. [Google Scholar] [CrossRef]
- Chi, H.; Zhang, Y.; Williams, P.N.; Lin, S.; Hou, Y.; Cai, C. In vitro model to assess arsenic bioaccessibility and speciation in cooked shrimp. J. Agric. Food Chem. 2018, 66, 4710–4715. [Google Scholar] [CrossRef] [Green Version]
- Lamsal, R.P.; Beauchemin, D. Estimation of the bio-accessible fraction of Cr, As, Cd and Pb in locally available bread using on-line continuous leaching method coupled to inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2015, 867, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.A.; Soares, T.R.P.; Mota, A.I.P.; Rosende, M.; Magalhaes, L.M.; Miro, M.; Segundo, M.A. Dynamic flow-through approach to evaluate readily bioaccessible antioxidants in solid food samples. Talanta 2017, 166, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Rosende, M.; Miró, M.; Cerdà, V. Fluidized-bed column method for automatic dynamic extraction and determination of trace element bioaccessibility in highly heterogeneous solid wastes. Anal. Chim. Acta 2010, 658, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Furtado, G.d.F.; Cunha, R.L. Bioaccessibility of lipophilic compounds vehiculated in emulsions: Choice of lipids and emulsifiers. J. Agric. Food Chem. 2019, 67, 13–18. [Google Scholar] [CrossRef]
- Cave, M.R.; Rosende, M.; Mounteney, I.; Gardner, A.; Miró, M. New insights into the reliability of automatic dynamic methods for oral bioaccessibility testing: A case study for BGS102 soil. Environ. Sci. Technol. 2016, 50, 9479–9486. [Google Scholar] [CrossRef] [Green Version]
- Rosende, M.; Miró, M. Recent trends in automatic dynamic leaching tests for assessing bioaccessible forms of trace elements in solid substrates. Trac-Trends Anal. Chem. 2013, 45, 67–78. [Google Scholar] [CrossRef]
- Chomchoei, R.; Miró, M.; Hansen, E.H.; Shiowatana, J. Sequential injection system incorporating a micro-extraction column for automatic fractionation of metal ions in solid samples: Comparison of the extraction profiles when employing uni-, bi-, and multi-bi-directional flow plus stopped-flow sequential extraction modes. Anal. Chim. Acta 2005, 536, 183–190. [Google Scholar]
- Fangueiro, D.; Bermond, A.; Santos, E.; Carapuça, H.; Duarte, A. Kinetic approach to heavy metal mobilization assessment in sediments: Choose of kinetic equations and models to achieve maximum information. Talanta 2005, 66, 844–857. [Google Scholar] [CrossRef]
- Labanowski, J.; Monna, F.; Bermond, A.; Cambier, P.; Fernandez, C.; Lamy, I.; van Oort, F. Kinetic extractions to assess mobilization of Zn, Pb, Cu, and Cd in a metal-contaminated soil: EDTA vs. citrate. Environ. Pollut. 2008, 152, 693–701. [Google Scholar] [CrossRef]
- Rosende, M.; Magalhaes, L.M.; Segundo, M.A.; Miro, M. Assessing oral bioaccessibility of trace elements in soils under worst-case scenarios by automated in-line dynamic extraction as a front end to inductively coupled plasma atomic emission spectrometry. Anal. Chim. Acta 2014, 842, 1–10. [Google Scholar] [CrossRef]
- Kersey, J.H.; Carter, R.A.; Buff, P.R.; Bauer, J.E. Natural pet food: A review of natural diets and their impact on canine and feline physiology. J. Anim. Sci. 2014, 92, 3781–3791. [Google Scholar]
- Top, A.; Cetinkaya, H. Zinc oxide and zinc hydroxide formation via aqueous precipitation: Effect of the preparation route and lysozyme addition. Mater. Chem. Phys. 2015, 167, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Hervera, M.; Baucells, M.D.; González, G.; Pérez, E.; Castrillo, C. Prediction of digestible protein content of dry extruded dog foods: Comparison of methods. J. Anim. Physiol. Anim. Nutr. 2009, 93, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Alborough, R.; Jones, L.; Davis, C.; Williams, C.; Gardner, D.S. Mineral analysis of complete dog and cat foods in the UK and compliance with European guidelines. Sci. Rep. 2017, 7, 17107. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds used in this work are available from the authors. |




| Sample 2 | Dynamic Extraction | Batch Extraction | |
|---|---|---|---|
| Gastric | Gastric + Intestinal | ||
| Sample #A | 107 ± 5 | 102 ± 7 | ND |
| Sample #B | 224 ± 4 | 194 ± 3 | ND |
| Sample #C | 222 ± 4 | 210 ± 9 | ND |
| Sample | Cumulative Bioaccessible Zn/mg kg−1 | A/mg kg−1 | B/min−1 | Total Amount of Zn/mg kg−1 | Bioaccessible Zn/% | Market Segment |
|---|---|---|---|---|---|---|
| Sample #1 | 186 ± 4 | 176 ± 3 | 0.290 ± 0.014 | 352 ± 40 | 52.8 | Premium |
| Sample #2 | 158 ± 1 | 160 ± 4 | 0.178 ± 0.010 | 295 ± 8 | 53.5 | Premium |
| Sample #3 | 120 ± 2 | 118 ± 2 | 0.163 ± 0.004 | 230 ± 57 | 52.3 | Economic |
| Sample #4 | 83 ± 1 | 83 ± 1 | 0.255 ± 0.008 | 169 ± 9 | 49.0 | Medium |
| Sample #5 | 161 ± 2 | 161 ± 1 | 0.236 ± 0.004 | 237 ± 10 | 68.1 | Premium |
| Sample #6 | 143 ± 0 | 149 ± 2 | 0.162 ± 0.004 | 204 ± 10 | 70.0 | Economic |
| Sample #7 | 317 ± 10 | 313 ± 5 | 0.233 ± 0.010 | 526 ± 14 | 60.2 | Premium |
| Sample #8 | 144 ± 1 | 143 ± 2 | 0.211 ± 0.007 | 286 ± 10 | 50.3 | Premium |
| Sample #9 | 191 ± 3 | 189 ± 3 | 0.182 ± 0.008 | 275 ± 5 | 69.4 | Medium |
| Sample #10 | 174 ± 1 | 174 ± 3 | 0.187 ± 0.008 | 285 ± 15 | 61.2 | Medium |
| Sample #11 | 222 ± 4 | 224 ± 5 | 0.184 ± 0.010 | 378 ± 23 | 58.7 | Premium |
| Sample#12 | 173 ± 3 | 160 ± 3 | 0.262 ± 0.013 | 263 ± 36 | 65.7 | Economic |
| Sample #13 | 224 ± 4 | 215 ± 3 | 0.239 ± 0.009 | 421 ± 30 | 53.3 | Medium |
| Sample #14 | 107 ± 5 | 106 ± 3 | 0.185 ± 0.011 | 212 ± 18 | 50.4 | Economic |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregório, B.J.R.; Pereira, A.M.; Fernandes, S.R.; Matos, E.; Castanheira, F.; Almeida, A.A.; Fonseca, A.J.M.; Cabrita, A.R.J.; Segundo, M.A. Flow-Based Dynamic Approach to Assess Bioaccessible Zinc in Dry Dog Food Samples. Molecules 2020, 25, 1333. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25061333
Gregório BJR, Pereira AM, Fernandes SR, Matos E, Castanheira F, Almeida AA, Fonseca AJM, Cabrita ARJ, Segundo MA. Flow-Based Dynamic Approach to Assess Bioaccessible Zinc in Dry Dog Food Samples. Molecules. 2020; 25(6):1333. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25061333
Chicago/Turabian StyleGregório, Bruno J. R., Ana Margarida Pereira, Sara R. Fernandes, Elisabete Matos, Francisco Castanheira, Agostinho A. Almeida, António J. M. Fonseca, Ana Rita J. Cabrita, and Marcela A. Segundo. 2020. "Flow-Based Dynamic Approach to Assess Bioaccessible Zinc in Dry Dog Food Samples" Molecules 25, no. 6: 1333. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25061333