Serjanic Acid Improves Immunometabolic Markers in a Diet-Induced Obesity Mouse Model
Abstract
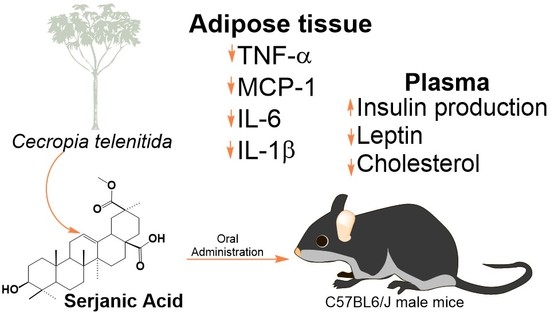
:1. Introduction
2. Results and Discussion
2.1. SA Extraction and Purification
2.2. Biological Activity Assessment
3. Materials and Methods
3.1. Instruments and Reagents
3.2. Plant Material Collection
3.3. Purification of SA from Cecropia telenitida Roots
3.4. Glucose and Insulin Tolerance Test in the Mouse Model of Insulin Resistance
3.5. Serum Biochemical Parameters Measurement
3.6. RNA Extraction and Real-Time PCR
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviation:
| Type 2 diabetes mellitus, T2DM; |
| cardiovascular disease, CVD; |
| pentacyclic triterpene, PT; |
| serjanic acid, SA; |
| high fat diet, HFD; |
| mertformin, Metf.; |
| triglycerides, TAG; |
| low density lipoprotein, LDL; |
| high density lipoprotein, HDL; |
| tumor necrosis factor-alpha, TNF-α; |
| monocyte chemoattractant protein-1, MCP-1; |
| interleukin-6, IL-6; |
| interleukin-1-beta, IL-1β; |
| acetyl-CoA carboxylase, ACC; |
| peroxisomal proliferation-activated receptor-alpha, PPAR-α; |
| peroxisome proliferator-activated receptor gamma coactivator-1, PGC-1; |
| carnitine palmitoyltransferase 1-A, Cpt1A. |
References
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S. Digging deeper into obesity. J. Clin. Investig. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strissel, K.J.; Stancheva, Z.; Miyoshi, H.; Perfield, J.W.; DeFuria, J.; Jick, Z.; Greenberg, A.S.; Obin, M.S. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumeng, C.N.; DeYoung, S.M.; Bodzin, J.L.; Saltiel, A.R. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 2007. [Google Scholar] [CrossRef] [Green Version]
- Aragão, D.M.O.; Guarize, L.; Lanini, J.; da Costa, J.C.; Garcia, R.M.G.; Scio, E. Hypoglycemic effects of Cecropia pachystachya in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 2010, 128, 629–633. [Google Scholar] [CrossRef]
- Revilla-Monsalve, M.C.; Andrade-Cetto, A.; Palomino-Garibay, M.A.; Wiedenfeld, H.; Islas-Andrade, S. Hypoglycemic effect of Cecropia obtusifolia Bertol aqueous extracts on type 2 diabetic patients. J. Ethnopharmacol. 2007, 111, 636–640. [Google Scholar] [CrossRef]
- Schinella, G.; Aquila, S.; Dade, M.; Giner, R.; Recio, M.D.C.; Spegazzini, E.; De Buschiazzo, P.; Tournier, H.; Ríos, J.L. Anti-inflammatory and apoptotic activities of pomolic acid isolated from Cecropia pachystachya. Planta Med. 2008, 74, 215–220. [Google Scholar] [CrossRef]
- Franco-Rosselli, P.; Berg, C.C. Distributional patterns of Cecropia (Cecropiaceae): A panbiogeographic analysis. Caldasia 1997, 19, 285–296. [Google Scholar]
- Costa, G.M.; Schenkel, E.P.; Reginatto, F.H. Chemical and pharmacological aspects of the genus Cecropia. Nat. Prod. Commun. 2011, 6, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Montoya, G.; Gutierrez, G.; D´vries, R.; Ellena, J.; Panay, A.J. Spergulagenic Acid A: Isolation and single crystal structure elucidation. J. Mol. Struct. 2018. [Google Scholar] [CrossRef]
- Mosquera, C.; Panay, A.J.; Montoya, G. Pentacyclic Triterpenes from Cecropia telenitida Can Function as Inhibitors of 11β-Hydroxysteroid Dehydrogenase Type 1. Molecules 2018, 23, 1444. [Google Scholar] [CrossRef] [Green Version]
- Montoya Peláez, G.L.; Sierra, J.A.; Alzate, F.; Holzgrabe, U.; Ramirez-Pineda, J.R. Pentacyclic triterpenes from Cecropia telenitida with immunomodulatory activity on dendritic cells. Braz. J. Pharm. 2013. [Google Scholar] [CrossRef] [Green Version]
- Siddique, H.R.; Saleem, M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [Green Version]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic triterpene bioavailability: An overview of in vitro and in vivo studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Sun, M.; Liu, Y.; Liang, J.; Wang, T.; Zhang, Z. Gymnemic Acid Alleviates Type 2 Diabetes Mellitus and Suppresses Endoplasmic Reticulum Stress in Vivo and in Vitro. J. Agric. Food Chem. 2019. [Google Scholar] [CrossRef]
- Mabhida, S.E.; Johnson, R.; Ndlovu, M.; Louw, J.; Opoku, A.; Mosa, R.A. Molecular basis of the anti-hyperglycemic activity of RA-3 in hyperlipidemic and streptozotocin-induced type 2 diabetes in rats. Diabetol. Metab. Syndr. 2019. [Google Scholar] [CrossRef]
- Khanra, R.; Bhattacharjee, N.; Dua, T.K.; Nandy, A.; Saha, A.; Kalita, J.; Manna, P.; Dewanjee, S. Taraxerol, a pentacyclic triterpenoid, from Abroma augusta leaf attenuates diabetic nephropathy in type 2 diabetic rats. Biomed. Pharmacother. 2017. [Google Scholar] [CrossRef]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. The effects of plant-derived oleanolic acid on selected parameters of glucose homeostasis in a diet-induced pre-diabetic rat model. Molecules 2018, 23, 794. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.; Kumar, P.; Deshmukh, R.R.; Bishayee, A.; Kumar, S. Pentacyclic triterpenes: New tools to fight metabolic syndrome. Phytomedicine 2018. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, A.M.; González-Ortiz, M.; Martínez-Abundis, E.; Acuña Ortega, N. Effect of Ursolic Acid on Metabolic Syndrome, Insulin Sensitivity, and Inflammation. J. Med. Food 2017. [Google Scholar] [CrossRef]
- Liu, J.; He, T.; Lu, Q.; Shang, J.; Sun, H.; Zhang, L. Asiatic acid preserves beta cell mass and mitigates hyperglycemia in streptozocin-induced diabetic rats. Diabetes Metab. Res. Rev. 2010. [Google Scholar] [CrossRef]
- Wu, J.B.; Kuo, Y.H.; Lin, C.H.; Ho, H.Y.; Shih, C.C. Tormentic Acid, a major component of suspension cells of eriobotrya japonica, suppresses high-fat diet-induced diabetes and hyperlipidemia by glucose transporter 4 and amp-activated protein kinase phosphorylation. J. Agric. Food Chem. 2014. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, W.; Zhou, Y.Y.; Zhang, Y.N.; Li, J.Y.; Hu, L.H.; Li, J. Corosolic acid stimulates glucose uptake via enhancing insulin receptor phosphorylation. Eur. J. Pharmacol. 2008. [Google Scholar] [CrossRef]
- Liu, J.; Sun, H.; Duan, W.; Mu, D.; Zhang, L. Maslinic Acid Reduces Blood Glucose in KK-Ay Mice. Biol. Pharm. Bull. 2007. [Google Scholar] [CrossRef] [Green Version]
- Ceballos, S.; Guillén, A.; Muñoz, D.L.; Castaño, A.; Echeverri, L.F.; Acín, S.; Balcázar, N. Immunometabolic regulation by triterpenes of Eucalyptus tereticornis in adipose tissue cell line models. Phytomedicine 2018. [Google Scholar] [CrossRef]
- Ramachandran, V.; Saravanan, R. Efficacy of asiatic acid, a pentacyclic triterpene on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin-induced diabetic rats. Phytomedicine 2013. [Google Scholar] [CrossRef]
- González-Garibay, A.S.; López-Vázquez, A.; García-Bañuelos, J.; Sánchez-Enríquez, S.; Sandoval-Rodríguez, A.S.; Del Toro Arreola, S.; Bueno-Topete, M.R.; Muñoz-Valle, J.F.; González Hita, M.E.; Domínguez-Rosales, J.A.; et al. Effect of Ursolic Acid on Insulin Resistance and Hyperinsulinemia in Rats with Diet-Induced Obesity: Role of Adipokines Expression. J. Med. Food 2019. [Google Scholar] [CrossRef]
- Kuljanabhagavad, T.; Thongphasuk, P.; Chamulitrat, W.; Wink, M. Triterpene saponins from Chenopodium quinoa Willd. Phytochemistry 2008. [Google Scholar] [CrossRef]
- Treyvaud, V.; Marston, A.; Dyatmiko, W.; Hostettmann, K. Molluscicidal saponins from Phytolacca icosandra. Phytochemistry 2000. [Google Scholar] [CrossRef]
- Bucar, F.; Wube, A.; Schmid, M. Natural product isolation-how to get from biological material to pure compounds. Nat. Prod. Rep. 2013. [Google Scholar] [CrossRef] [Green Version]
- Antunes, L.C.; Elkfury, J.L.; Jornada, M.N.; Foletto, K.C.; Bertoluci, M.C. Validation of HOMA-IR in a model of insulin-resistance induced by a high-fat diet in Wistar rats. Arch. Endocrinol. Metab. 2016. [Google Scholar] [CrossRef] [Green Version]
- Castellano, J.M.; Guinda, A.; Delgado, T.; Rada, M.; Cayuela, J.A. Biochemical Basis of the Antidiabetic Activity of Oleanolic Acid and Related Pentacyclic Triterpenes. Diabetes 2013, 62, 1791–1799. [Google Scholar] [CrossRef] [Green Version]
- Gilon, P.; Henquin, J.C. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr. Rev. 2001, 22, 565–604. [Google Scholar] [CrossRef] [Green Version]
- Whalley, N.M.; Pritchard, L.E.; Smith, D.M.; White, A. Processing of proglucagon to GLP-1 in pancreatic α-cells: Is this a paracrine mechanism enabling GLP-1 to act on β-cells? J. Endocrinol. 2011, 211, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Genet, C.; Strehle, A.; Thomas, C.; Lobstein, A.; Wagner, A.; Mioskowski, C.; Auwerx, J.; Saladin, R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun. 2007, 362, 793–798. [Google Scholar] [CrossRef]
- Mosa, R.A.; Cele, N.D.; Mabhida, S.E.; Shabalala, S.C.; Penduka, D.; Opoku, A.R. In vivo antihyperglycemic activity of a lanosteryl triterpene from Protorhus longifolia. Molecules 2015, 20, 13374–13383. [Google Scholar] [CrossRef]
- Mabhida, S.E.; Mosa, R.A.; Penduka, D.; Osunsanmi, F.O.; Dludla, P.V.; Djarova, T.G.; Opoku, A.R. A lanosteryl triterpene from protorhus longifolia improves glucose tolerance and pancreatic beta cell ultrastructure in type 2 diabetic rats. Molecules 2017, 22, 1252. [Google Scholar] [CrossRef] [Green Version]
- Mabhida, S.E.; Dludla, P.V.; Johnson, R.; Ndlovu, M.; Louw, J.; Opoku, A.R.; Mosa, R.A. Protective effect of triterpenes against diabetes-induced β-cell damage: An overview of in vitro and in vivo studies. Pharmacol. Res. 2018. [Google Scholar] [CrossRef]
- Castro, A.J.G.; Cazarolli, L.H.; De Carvalho, F.K.; Da Luz, G.; Altenhofen, D.; Dos Santos, A.R.S.; Pizzolatti, M.G.; Silva, F.R.M.B. Acute effect of 3β-hidroxihop-22(29)ene on insulin secretion is mediated by GLP-1, potassium and calcium channels for the glucose homeostasis. J. Steroid Biochem. Mol. Biol. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.; Li, W.; Xiao, D.; Wei, S.; Cui, W.; Chen, W.; Hu, Y.; Bi, X.; Kim, Y.; Li, J.; et al. Compound K, a final intestinal metabolite of ginsenosides, enhances insulin secretion in MIN6 pancreatic β-cells by upregulation of GLUT2. Fitoterapia 2013. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.M.; Quintanilla-Fend, L.; Bettio, S.; Jauch, J.; Scior, T.; Scherbaum, W.A.; Ammon, H.P.T. 11-keto-β-boswellic acids prevent development of autoimmune reactions, insulitis and reduce hyperglycemia during induction of multiple low-dose streptozotocin (MLD-STZ) diabetes in mice. Horm. Metab. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.A.; Frota, J.T.; Arruda, B.R.; De Melo, T.S.; Da Silva, A.A.D.C.A.; Brito, G.A.D.C.; Chaves, M.H.; Rao, V.S. Antihyperglycemic and hypolipidemic effects of α,β-amyrin, a triterpenoid mixture from Protium heptaphyllum in mice. Lipids Health Dis. 2012. [Google Scholar] [CrossRef] [Green Version]
- Manna, P.; Sinha, M.; Sil, P.C. Protective role of arjunolic acid in response to streptozotocin-induced type-I diabetes via the mitochondrial dependent and independent pathways. Toxicology 2009. [Google Scholar] [CrossRef]
- Weisberg, S.; Leibel, R.; Tortoriello, D.V. Proteasome inhibitors, including curcumin, improve pancreatic β-cell function and insulin sensitivity in diabetic mice. Nutr. Diabetes 2016. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Pan, J.H.; Cho, H.T.; Kim, Y.J. Black ginseng extract counteracts streptozotocin-induced diabetes in mice. PLoS ONE 2016. [Google Scholar] [CrossRef] [Green Version]
- Reeds, D.N.; Patterson, B.W.; Okunade, A.; Holloszy, J.O.; Polonsky, K.S.; Klein, S. Ginseng and ginsenoside re do not improve β-cell function or insulin sensitivity in overweight and obese subjects with impaired glucose tolerance or diabetes. Diabetes Care 2011. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, E.J.; Leroith, D.; Karnieli, E. Insulin resistance in obesity as the underlying cause for the metabolic syndrome. Mt. Sinai J. Med. 2010. [Google Scholar] [CrossRef]
- Dominguez, E.; Galmozzi, A.; Chang, J.W.; Hsu, K.-L.; Pawlak, J.; Li, W.; Godio, C.; Thomas, J.; Partida, D.; Niessen, S.; et al. Integrated phenotypic and activity-based profiling links Ces3 to obesity and diabetes. Nat. Chem. Biol. 2014, 10, 113–121. [Google Scholar] [CrossRef]
- Song, P.-F.; Zhu, Y.-D.; Ma, H.-Y.; Wang, Y.-N.; Wang, D.-D.; Zou, L.-W.; Ge, G.-B.; Yang, L. Discovery of natural pentacyclic triterpenoids as potent and selective inhibitors against human carboxylesterase 1. Fitoterapia 2019, 137, 104199. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.-W.; Dou, T.-Y.; Wang, P.; Lei, W.; Weng, Z.-M.; Hou, J.; Wang, D.-D.; Fan, Y.-M.; Zhang, W.-D.; Ge, G.-B.; et al. Structure-Activity Relationships of Pentacyclic Triterpenoids as Potent and Selective Inhibitors against Human Carboxylesterase 1. Front. Pharmacol. 2017, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Lee, G.Y.; Kim, J.; Lee, Y.M.; Kim, J.M.; Kim, Y.S.; Kim, J.S. A new pancreatic lipase inhibitor isolated from the roots of Actinidia arguta. Arch. Pharm. Res. 2008, 31, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Xie, Y.; Asao, Y.; Okamoto, M.; Yamashita, C.; Muraoka, O.; Matsuda, H.; Pongpiriyadacha, Y.; Yuan, D.; Yoshikawa, M. Oleanane-type triterpene oligoglycosides with pancreatic lipase inhibitory activity from the pericarps of Sapindus rarak. Phytochemistry 2009, 70, 1166–1172. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.S.; Kim, C.-S.; Kim, J.S. Betulinic Acid has an Inhibitory Effect on Pancreatic Lipase and Induces Adipocyte Lipolysis. Phyther. Res. 2012, 26, 1103–1106. [Google Scholar] [CrossRef]
- de la Garza, A.L.; Milagro, F.I.; Boque, N.; Campión, J.; Martínez, J.A. Natural Inhibitors of Pancreatic Lipase as New Players in Obesity Treatment. Planta Med. 2011, 77, 773–785. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.H.; Lin, C.H.; Shih, C.C.; Yang, C.S. Antcin K, a Triterpenoid Compound from Antrodia camphorata, Displays Antidiabetic and Antihyperlipidemic Effects via Glucose Transporter 4 and AMP-Activated Protein Kinase Phosphorylation in Muscles. Evid. Based Complement. Altern. Med. 2016. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.H.; Kuo, Y.H.; Shih, C.C. Antidiabetic and hypolipidemic activities of eburicoic acid, a triterpenoid compound from: Antrodia camphorata, by regulation of Akt phosphorylation, gluconeogenesis, and PPARα in streptozotocin-induced diabetic mice. RSC Adv. 2018. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.H.; Lin, C.H.; Shih, C.C. Antidiabetic and Antihyperlipidemic Properties of a Triterpenoid Compound, Dehydroeburicoic Acid, from Antrodia camphorata in Vitro and in Streptozotocin-Induced Mice. J. Agric. Food Chem. 2015. [Google Scholar] [CrossRef]
- Santos-Alvarez, J.; Goberna, R.; Sánchez-Margalet, V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell. Immunol. 1999. [Google Scholar] [CrossRef]
- La Cava, A.; Matarese, G. The weight of leptin in immunity. Nat. Rev. Immunol. 2004, 4, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Faouzi, M.; Leshan, R.; Björnholm, M.; Hennessey, T.; Jones, J.; Münzberg, H. Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology 2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yizhen, W. The Effect of Triterpene Saponins on Hypothalamic and Cortical Leptin Sensitivity in Obese Mice; University of Wollongong: Wollongong, Australia, 2018. [Google Scholar]
- Patil, K.R.; Mohapatra, P.; Patel, H.M.; Goyal, S.N.; Ojha, S.; Kundu, C.N.; Patil, C.R. Pentacyclic triterpenoids inhibit ikkβmediated activation of nf-κb pathway: In silico and in vitro evidences. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swaroop, J.J.; Rajarajeswari, D.; Naidu, J.N. Association of TNF-α with insulin resistance in type 2 diabetes mellitus. Indian J. Med. Res. 2012, 135, 127–130. [Google Scholar] [CrossRef]
- Arkan, M.C.; Hevener, A.L.; Greten, F.R.; Maeda, S.; Li, Z.-W.; Long, J.M.; Wynshaw-Boris, A.; Poli, G.; Olefsky, J.; Karin, M.; et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef]
- Nieto-Vazquez, I.; Fernández-Veledo, S.; Krämer, D.K.; Vila-Bedmar, R.; Garcia-Guerra, L.; Lorenzo, M. Insulin resistance associated to obesity: The link TNF-alpha. Arch. Physiol. Biochem. 2008, 114, 183–194. [Google Scholar] [CrossRef]
- Ogihara, T.; Asano, T.; Katagiri, H.; Sakoda, H.; Anai, M.; Shojima, N.; Ono, H.; Fujishiro, M.; Kushiyama, A.; Fukushima, Y.; et al. Oxidative stress induces insulin resistance by activating the nuclear factor-kappa B pathway and disrupting normal subcellular distribution of phosphatidylinositol 3-kinase. Diabetologia 2004, 47, 794–805. [Google Scholar] [CrossRef]
- Decara, J.; Arrabal, S.; Beiroa, D.; Rivera, P.; Vargas, A.; Serrano, A.; Pavón, F.J.; Ballesteros, J.; Dieguez, C.; Nogueiras, R.; et al. Antiobesity efficacy of GLP-1 receptor agonist liraglutide is associated with peripheral tissue-specific modulation of lipid metabolic regulators. BioFactors 2016. [Google Scholar] [CrossRef]
- Patiny, L.; Borel, A. ChemCalc: A Building Block for Tomorrow’s Chemical Infrastructure. J. Chem. Inf. Model. 2013, 53, 1223–1228. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, G.; Giraldo-Dávila, D.; Combariza, M.Y.; Holzgrabe, U.; Tabares-Guevara, J.H.; Ramírez-Pineda, J.R.; Acín, S.; Muñoz, D.L.; Montoya, G.; Balcazar, N. Serjanic Acid Improves Immunometabolic Markers in a Diet-Induced Obesity Mouse Model. Molecules 2020, 25, 1486. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071486
Gutiérrez G, Giraldo-Dávila D, Combariza MY, Holzgrabe U, Tabares-Guevara JH, Ramírez-Pineda JR, Acín S, Muñoz DL, Montoya G, Balcazar N. Serjanic Acid Improves Immunometabolic Markers in a Diet-Induced Obesity Mouse Model. Molecules. 2020; 25(7):1486. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071486
Chicago/Turabian StyleGutiérrez, Gustavo, Deisy Giraldo-Dávila, Marianny Y. Combariza, Ulrike Holzgrabe, Jorge Humberto Tabares-Guevara, José Robinson Ramírez-Pineda, Sergio Acín, Diana Lorena Muñoz, Guillermo Montoya, and Norman Balcazar. 2020. "Serjanic Acid Improves Immunometabolic Markers in a Diet-Induced Obesity Mouse Model" Molecules 25, no. 7: 1486. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071486