Norhierridin B, a New Hierridin B-Based Hydroquinone with Improved Antiproliferative Activity
Abstract
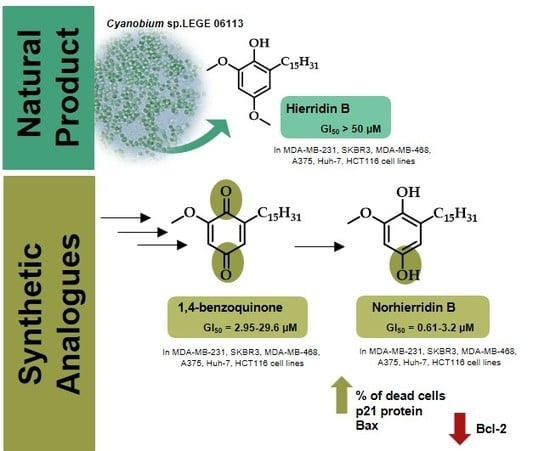
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Biological Activity
3. Material and Methods
3.1. Synthesis
3.1.1. Synthesis of 2-(1-hydroxypentadecyl)-6-methoxyphenol (7)
3.1.2. Synthesis of 2-methoxy-6-pentadecylphenol (8)
3.1.3. Synthesis of 2-methoxy-6-pentadecyl-1,4-benzoquinone (9)
3.1.4. Synthesis of 2-methoxy-6-pentadecylbenzene-1,4-diol (10).
3.2. Biological Activity
3.2.1. Human Cell Lines and Growth Conditions
3.2.2. Sulforhodamine B (SRB) Assay
3.2.3. Colony Formation Assay
3.2.4. Cell Viability Assay
3.2.5. Cell Cycle Analysis
3.2.6. Western Blot Analysis
3.2.7. Statistical analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- García, P.; Hernández, Á.; San Feliciano, A.; Castro, M. Bioactive Prenyl-and Terpenyl-Quinones/Hydroquinones of Marine Origin. Mar. Drugs. 2018, 16, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asche, C. Antitumour quinones. Mini Rev. Med. Chem. 2005, 5, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Sunassee, S.N.; Davies-Coleman, M.T. Cytotoxic and antioxidant marine prenylated quinones and hydroquinones. Nat. Prod. Rep. 2012, 29, 513–535. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-S.; Lin, Y.-J.; Lee, S.-J.; Yang, C.-W.; Lin, W.-Y.; Tsai, I.-L.; Chen, I.-S. Cytotoxic alkyl benzoquinones and alkyl phenols from Ardisia virens. Phytochemistry. 2009, 70, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Cheng-Sánchez, I.; Torres-Vargas, J.A.; Martínez-Poveda, B.; Guerrero-Vásquez, G.A.; Medina, M.Á.; Sarabia, F.; Quesada, A.R. Synthesis and Antitumor Activity Evaluation of Compounds Based on Toluquinol. Mar. Drugs. 2019, 17, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leao, P.N.; Costa, M.; Ramos, V.; Pereira, A.R.; Fernandes, V.C.; Domingues, V.F.; Gerwick, W.H.; Vasconcelos, V.M.; Martins, R. Antitumor activity of hierridin B, a cyanobacterial secondary metabolite found in both filamentous and unicellular marine strains. PloS ONE. 2013, 8, e69562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, A.G.; Barrera, J.B.; Perez, E.M.R. Synthesis of hierridin, a phenol from the lichen, Ramalina hierrensis. Phytochemistry. 1992, 31, 1436–1439. [Google Scholar] [CrossRef]
- Liu, X.; Lu, G.; Guo, Y.; Guo, Y.; Wang, Y.; Wang, X. Catalytic transfer hydrogenolysis of 2-phenyl-2-propanol over palladium supported on activated carbon. J. Mol. Catal. A: Chem. 2006, 252, 176–180. [Google Scholar] [CrossRef]
- Feng, J.; Yang, C.; Zhang, D.; Wang, J.; Fu, H.; Chen, H.; Li, X. Catalytic transfer hydrogenolysis of α-methylbenzyl alcohol using palladium catalysts and formic acid. Appl. Catal. A: Gen. 2009, 354, 38–43. [Google Scholar] [CrossRef]
- Sawadjoon, S.; Lundstedt, A.; Samec, J.S. Pd-catalyzed transfer hydrogenolysis of primary, secondary, and tertiary benzylic alcohols by formic acid: A mechanistic study. Acs Catal. 2013, 3, 635–642. [Google Scholar] [CrossRef]
- König, W.A.; Faasch, H.; Heitsch, H.; Colberg, C.; Hausen, B.M. Synthese von seitenkettenmodifizierten Analogen des Allergens Primin/Synthesis of Side-Chain-Modified Analogues of the Allergen Primin. Z. Naturforsch. B. 1993, 48, 387–393. [Google Scholar] [CrossRef]
- Davis, C.J.; Hurst, T.E.; Jacob, A.M.; Moody, C.J. Microwave-mediated Claisen rearrangement followed by phenol oxidation: A simple route to naturally occurring 1, 4-benzoquinones. The first syntheses of verapliquinones A and B and panicein A. J. Org. Chem. 2005, 70, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Sampaio-Dias, I.E.; Castelo-Branco, R.; Scharfenstein, H.; Rezende de Castro, R.; Silva, A.; Schneider, M.P.C.; Araújo, M.J.O.; Martins, R.R.; Domingues, V.F. Structure of Hierridin C, Synthesis of Hierridins B and C, and Evidence for Prevalent Alkylresorcinol Biosynthesis in Picocyanobacteria. J. Nat. Prod. 2019, 82, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, J.; Haddad, F.G.; Eid, R.; Lambertini, M.; Kourie, H.R. Triple-negative breast cancer: Current perspective on the evolving therapeutic landscape. Int. J. Womens Health. 2019, 11, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrin, D.; Armarego, W. Purification of Organic Chemicals, 3nd ed.; Pergamon Press: Oxford, UK, 1988; Volume 3, p. 65. [Google Scholar]
- Soares, J.; Espadinha, M.; Raimundo, L.; Ramos, H.; Gomes, A.S.; Gomes, S.; Loureiro, J.B.; Inga, A.; Reis, F.; Gomes, C. DIMP 53-1: A novel small-molecule dual inhibitor of p53–MDM 2/X interactions with multifunctional p53-dependent anticancer properties. Mol. Oncol. 2017, 11, 612–627. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 7, 9 and 10 are available from the authors. |




| GI50 (μM) | ||||||
|---|---|---|---|---|---|---|
| Cell line/Compound | 6 | 7 | 8 | 9 | 10 | Etoposide |
| MDA-MB-231 | >50 | 9.65 ± 0.15 | 28.8 ± 3.3 | 5.83 ± 1.10 | 0.61 ± 0.07 | 5.06 ± 1.24 |
| SKBR3 | >50 | 14.0 ± 0.0 | >50 | 4.35 ± 0.15 | 0.77 ± 0.06 | 4.77 ± 1.09 |
| MDA-MB-468 | >50 | 7.85 ± 2.15 | 31.00 ± 7.00 | 6.65 ± 0.90 | 0.68 ± 0.13 | 2.14 ± 0.36 |
| A375 | >50 | 16.0 ± 1.7 | 32.9 ± 4.2 | 20.6 ± 1.9 | 2.0 ± 0.4 | 0.9 ± 0.07 |
| Huh-7 | >50 | 27.5 ± 0.5 | >50 | 2.95 ± 0.15 | 0.61 ± 0.03 | 3.43 ± 0.85 |
| HCT116 | >50 | 26.5 ± 0.5 | 26.0 ± 3.2 | 29.6 ± 0.5 | 3.2 ± 0.6 | 0.67 ± 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão, P.; Moreira, J.; Almeida, J.; Nazareth, N.; Sampaio-Dias, I.E.; Vasconcelos, V.; Martins, R.; Leão, P.; Pinto, M.; Saraíva, L.; et al. Norhierridin B, a New Hierridin B-Based Hydroquinone with Improved Antiproliferative Activity. Molecules 2020, 25, 1578. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071578
Brandão P, Moreira J, Almeida J, Nazareth N, Sampaio-Dias IE, Vasconcelos V, Martins R, Leão P, Pinto M, Saraíva L, et al. Norhierridin B, a New Hierridin B-Based Hydroquinone with Improved Antiproliferative Activity. Molecules. 2020; 25(7):1578. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071578
Chicago/Turabian StyleBrandão, Pedro, Joana Moreira, Joana Almeida, Nair Nazareth, Ivo E. Sampaio-Dias, Vitor Vasconcelos, Rosário Martins, Pedro Leão, Madalena Pinto, Lucília Saraíva, and et al. 2020. "Norhierridin B, a New Hierridin B-Based Hydroquinone with Improved Antiproliferative Activity" Molecules 25, no. 7: 1578. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071578