Determination of Albumin, Glucose, and Creatinine Employing a Single Sequential Injection Lab-at-Valve with Mono-Segmented Flow System Enabling In-Line Dilution, In-Line Single-Standard Calibration, and In-Line Standard Addition
Abstract
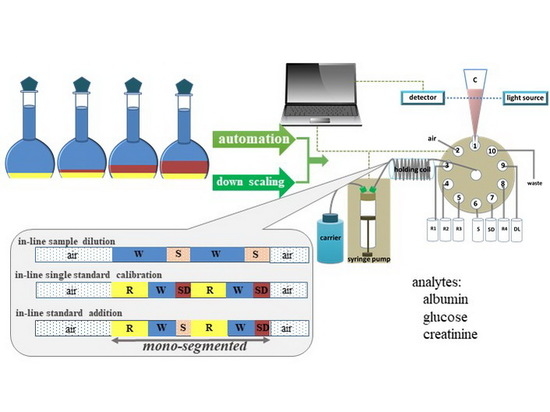
:1. Introduction
2. Results and Discussion
2.1. Determination of Albumin
2.2. Determination of Glucose
2.3. Determination of Creatinine
2.4. Preliminary Work in Application to a Real Sample
3. Experimental
3.1. Reagents and Chemicals
3.2. The Instrument Setup
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Assali, M.; Raimundo, J.; Facchin, I. Simultaneous multiple injection to perform titration and standard addition in monosegmented flow analysis. J. Autom. Methods Manag. Chem. 2001, 23, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.S.P.; Masini, J.C. Exploiting monosegmented flow analysis to perform in-line standard additions using a single stock standard solution in spectrophotometric sequential injection procedures. Anal. Chim. Acta 2002, 466, 345–352. [Google Scholar] [CrossRef]
- Silva, M.S.P.; Galhardo, C.X.; Masini, J.C. Application of sequential injection-monosegmented flow analysis (SI-MSFA) to spectrophotometric determination of sulfide in simulated waters samples. Talanta 2003, 60, 45–52. [Google Scholar] [CrossRef]
- Dos Santos, L.B.O.; Silva, M.S.P.; Masini, J.C. Developing a sequential injection-square wave voltammetry (SI-SWV) method for determination of atrazine using a hanging mercury drop electrode. Anal. Chim. Acta 2005, 528, 21–27. [Google Scholar] [CrossRef]
- Dos Santos, L.B.O.; Masini, J.C. Determination of picloram in natural waters employing sequential injection square wave voltammetry using the hanging mercury drop electrode. Talanta 2007, 72, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Kozak, J.; Wójtowicz, M.; Wróbel, A.; Kościelniak, P. Novel approach to calibration by the complementary dilution method with the use of a monosegmented sequential injection system. Talanta 2008, 77, 587–592. [Google Scholar] [CrossRef]
- Dos Santos, L.B.O.; Masini, J.C. Square wave adsorptive cathodic stripping voltammetry automated by sequential injection analysis. Potentialities and limitations exemplified by the determination of methyl parathion in water samples. Anal. Chim. Acta 2008, 606, 209–216. [Google Scholar] [CrossRef]
- Siriangkhawut, W.; Grudpan, K.; Jakmunee, J. Sequential injection anodic stripping voltammetry with monosegmented flow and in-line UV digestion for determination of Zn(II), Cd(II), Pb(II) and Cu(II) in water samples. Talanta 2011, 84, 1366–1373. [Google Scholar] [CrossRef]
- Kozak, J.; Wójtowicz, M.; Gawenda, N.; Kościelniak, P. An automatic system for acidity determination based on sequential injection titration and the monosegmented flow approach. Talanta 2011, 84, 1379–1383. [Google Scholar] [CrossRef]
- Kochana, J.; Kozak, J.; Skrobisz, A.; Woźniakiewicz, M. Tyrosinase biosensor for benzoic acid inhibition-based determination with the use of a flow-batch monosegmented sequential injection system. Talanta 2012, 96, 147–152. [Google Scholar] [CrossRef]
- Barreto, I.S.; Andrade, S.I.E.; Lima, M.B.; Silva, E.C.; Araújo, M.C.U.; Almeida, L.F. A monosegmented flow-batch system for slow reaction kinetics: Spectrophotometric determination of boron in plants. Talanta 2012, 94, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Khanhuathon, Y.; Siriangkhawut, W.; Chantiratikul, P.; Grudpan, K. Flow-Batch Method with a Sequential Injection System for Spectrophotometric Determination of Selenium(IV) in Selenium-Enriched Yeast Using o-Phenylenediamine. Anal. Lett. 2013, 46, 1779–1792. [Google Scholar] [CrossRef]
- Khanhuathon, Y.; Siriangkhawut, W.; Chantiratikul, P.; Grudpan, K. Spectrophotometric method for determination of aluminium content in water and beverage samples employing flow-batch sequential injection system. J. Food Compos. Anal. 2015, 41, 45–53. [Google Scholar] [CrossRef]
- Grudpan, K. Some recent developments on cost-effective flow-based analysis. Talanta 2004, 64, 1084–1090. [Google Scholar] [CrossRef]
- Sakai, T.; Kito, Y.; Teshima, N.; Katoh, S.; Watla-iad; Grudpan, K. Spectrophotometric Flow Injection Analysis of Protein in Urine Using Tetrabromophenolphthalein Ethyl Ester and Triton X-100. J. Flow Injection Anal. 2007, 24, 23–26. [Google Scholar]
- Watla-iad, K.; Sakai, T.; Teshima, N.; Katoh, S.; Grudpan, K. Successive determination of urinary protein and glucose using spectrophotometric sequential injection method. Anal. Chim. Acta 2007, 604, 139–146. [Google Scholar]
- Sakai, T.; Ohta, H.; Ohno, N.; Imai, J. Routine assay of creatinine in newborn baby urine by spectrophotometric flow-injection analysis. Anal. Chim. Acta 1995, 308, 446–450. [Google Scholar] [CrossRef]
- Siangproh, W.; Teshima, N.; Sakai, T.; Katoh, S.; Chailapakul, O. Alternative method for measurement of albumin/creatinine ratio using spectrophotometric sequential injection analysis. Talanta 2009, 79, 1111–1117. [Google Scholar] [CrossRef]
- Songjaroen, T.; Maturos, T.; Sappat, A.; Tuantranont, A.; Laiwattanapaisal, W. Portable microfluidic system for determination of urinary creatinine. Anal. Chim. Acta 2009, 647, 78–83. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2005; ISBN 9780131291928. [Google Scholar]
- Odebunmi, E.O.; Owalude, S.O. Kinetic and thermodynamic studies of glucose oxidase catalysed oxidation reaction of glucose. J. Appl. Sci. Environ. Manag. December 2007, 11, 95–100. [Google Scholar]
- Paengnakorn, P.; Chanpaka, S.; Watla-iad, K.; Wongwilai, W.; Grudpan, K. Towards Green Titration: Downscaling the Sequential Injection Analysis Lab-at-valve Titration System with the Stepwise Addition of a Titrant. Anal. Sci. 2018, 35, 219–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: The urine samples are not available from the authors. |






| No. | Analyte(s)/Sample (s) | Detection/Technique | Reagent | Role of Mono-Segmented | Ref. | ||
|---|---|---|---|---|---|---|---|
| Sample Conditioning (Inline Dilution) | Inline Single Std. Calibration | Inline Std. Addition | |||||
| 1 | Fe(II) in pharmaceutical preparations, Cr(VI) in natural water and domestic waste water samples | solution handing for spectrophotometric determination (Fe(II) and Cr(VI)) | KMnO4 for Fe(II) and diphenylcarbazide for Cr(VI) | X | [1] | ||
| 2 | Fe(II) in anti-anemic medicine | spectrophotometric determination of Fe(II) | 1,10-phenanthroline | X | X | X | [2] |
| 3 | sulfide in waters | spectrophotometric detection | Fe(III) and N,N-dimethyl-p-phenylene diamine hydrochloride | X | X | [3] | |
| 4 | atrazine | voltammetric detection | X | X | [4] | ||
| 5 | picloram in natural waters | voltammetric detection | X | X | [5] | ||
| 6 | Mg, Ca in water sample | flame atomic absorption spectrometric detection | X | X | [6] | ||
| 7 | methyl parathion in water sample | voltammetric detection | X | X | [7] | ||
| 8 | Zn(II), Cd(II), Pb(II) and Cu(II) in water samples | voltammetric dectection | X | X | X | [8] | |
| 9 | Al in water and beverage samples | tritrarion with spectrophotometric determination | sodium hydroxide as a titrant and phenolphthalein or thymolphthalein indicator | X | [9] | ||
| 10 | benzoic acid in a real beverage sample | amperometric detection | biosensor is based on the inhibition effect of benzoic acid on the biocatalytic activity of tyrosinase, polyphenol oxidase. | X | X | X | [10] |
| 11 | B in plants | spectrophotometric detection | azomethine-H | X | [11] | ||
| 12 | Se (IV) in raw Se-enriched yeast | spectrophotometric detection | o-pheneylenediamine | X | X | [12] | |
| 13 | Al in water and beverage samples. | spectrophotometric detection | Eriochrome cyanine R | X | X | [13] | |
| Biomarker | Concentration (mg/dL) |
|---|---|
| Albumin | 1.8 |
| Glucose | not detectable ** |
| creatinine | 129 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiwfo, K.; Wongwilai, W.; Sakai, T.; Teshima, N.; Grudpan, K. Determination of Albumin, Glucose, and Creatinine Employing a Single Sequential Injection Lab-at-Valve with Mono-Segmented Flow System Enabling In-Line Dilution, In-Line Single-Standard Calibration, and In-Line Standard Addition. Molecules 2020, 25, 1666. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071666
Kiwfo K, Wongwilai W, Sakai T, Teshima N, Grudpan K. Determination of Albumin, Glucose, and Creatinine Employing a Single Sequential Injection Lab-at-Valve with Mono-Segmented Flow System Enabling In-Line Dilution, In-Line Single-Standard Calibration, and In-Line Standard Addition. Molecules. 2020; 25(7):1666. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071666
Chicago/Turabian StyleKiwfo, Kanokwan, Wasin Wongwilai, Tadao Sakai, Norio Teshima, and Kate Grudpan. 2020. "Determination of Albumin, Glucose, and Creatinine Employing a Single Sequential Injection Lab-at-Valve with Mono-Segmented Flow System Enabling In-Line Dilution, In-Line Single-Standard Calibration, and In-Line Standard Addition" Molecules 25, no. 7: 1666. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071666