Induced Wide Nematic Phase by Seven-Ring Supramolecular H-Bonded Systems: Experimental and Computational Evaluation
Abstract
:1. Introduction
2. Experimental
Preparation of 2:1 SMHB Complexes
3. Results and Discussion
3.1. FT–IR Characterizations of 2:1 SMHB Complexes
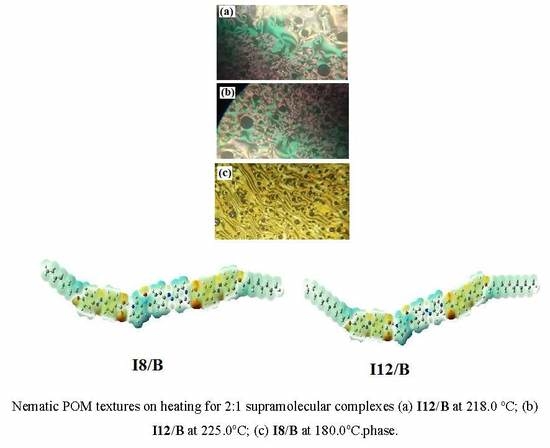
3.2. Mesomorphic Study of 2:1 SMHBLC Complexes
3.3. DFT Calculations
3.3.1. Molecular Geometries
3.3.2. Effect of the H-Bonding and π–π Stacking on the Mesophase Behavior
3.3.3. Thermal Parameters
3.3.4. Frontier Molecular Orbitals, Dipole Moment and Polarizability
3.3.5. Molecular Electrostatic Potential (MEP)
3.4. Photophysical Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hagar, M.; Ahmed, H.; Alhaddad, O. DFT calculations and mesophase study of coumarin esters and its azoesters. Crystals 2018, 8, 359. [Google Scholar] [CrossRef] [Green Version]
- Hagar, M.; Ahmed, H.; Alhaddad, O. Experimental and theoretical approaches of molecular geometry and mesophase behaviour relationship of laterally substituted azopyridines. Liq. Cryst. 2019, 46, 1440–1451. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Alaasar, M.A.; Salem, R.A. Supramolecular liquid crystals in binary and ternary systems. Thermochim. Acta 2011, 517, 63–73. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Alhaddad, O.A. Phase behavior and DFT calculations of laterally methyl supramolecular hydrogen-bonding complexes. Crystals 2019, 9, 133. [Google Scholar] [CrossRef] [Green Version]
- Hagar, M.; Ahmed, H.; Saad, G. New calamitic thermotropic liquid crystals of 2-hydroxypyridine ester mesogenic core: Mesophase behaviour and DFT calculations. Liq. Cryst. 2020, 47, 114–124. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Alhaddad, O. New chair shaped supramolecular complexes-based aryl nicotinate derivative; mesomorphic properties and DFT molecular geometry. RSC Adv. 2019, 9, 16366–16374. [Google Scholar] [CrossRef] [Green Version]
- Ong, L.-K.; Ha, S.-T.; Yeap, G.-Y.; Lin, H.-C. Heterocyclic pyridine-based liquid crystals: Synthesis and mesomorphic properties. Liq. Cryst. 2018, 45, 1574–1584. [Google Scholar] [CrossRef]
- Foo, K.-L.; Ha, S.-T.; Yeap, G.; Lee, S. Mesomorphic behaviors of a series of heterocyclic thiophene-imine-ester-based liquid crystals. Phase Transit. 2018, 91, 509–520. [Google Scholar] [CrossRef]
- Foo, K.-L.; Ha, S.-T.; Yeap, G.-Y.; Lin, H.-C. Mesomorphic behaviors of a series of heterocyclic benzothiazole-imine-ester-based liquid crystals. Phase Transit. 2019, 92, 87–99. [Google Scholar] [CrossRef]
- Nafee, S.S.; Ahmed, H.; Hagar, M. Theoretical, experimental and optical study of new thiophene-based liquid crystals and their positional isomers. Liq. Cryst. 2020, 1–12. [Google Scholar] [CrossRef]
- Du, S.; Zhang, M.; Chen, P.; Dang, J.; Gao, A.; Du, W.; Chen, X.; An, Z. Improved mesomorphic behaviour and large birefringence of fluorinated liquid crystals containing ethynyl and 1-methyl-1h-benzimidazole moieties. Liq. Cryst. 2020. [Google Scholar] [CrossRef]
- Ghosh, T.; Lehmann, M. Recent advances in heterocycle-based metal-free calamitics. J. Mater. Chem. C 2017, 5, 12308–12337. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Tang, B.-C.; Zhang, P.; Li, M.; Tian, W.-J. Synthesis and characterization of 1,3,4-oxadiazole derivatives containing alkoxy chains with different lengths. J. Mol. Struct. 2007, 846, 55–64. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Quan, M.; Zhang, L.; Yang, H.; Lu, Y. Photothermal effect of azopyridine compounds and their applications. RSC Adv. 2015, 5, 4675–4680. [Google Scholar] [CrossRef]
- Garcia-Amorós, J.; Reig, M.; Cuadrado, A.; Ortega, M.; Nonell, S.; Velasco, D. A photoswitchable bis-azo derivative with a high temporal resolution. Chem. Commun. 2014, 50, 11462–11464. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, H. Different morphologies of self-assembled nanofibers fabricated with amphiphilic low-molecular-weight azopyridinium salts. RSC Adv. 2013, 3, 22155–22159. [Google Scholar] [CrossRef]
- Zhou, W.; Kobayashi, T.; Zhu, H.; Yu, H. Electrically conductive hybrid nanofibers constructed with two amphiphilic salt components. Chem. Commun. 2011, 47, 12768–12770. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, R.; Jackson, J.K.; Chiao, M.; Yu, H. Janus ultrathin film from multi-level self-assembly at air–water interfaces. Chem. Commun. 2014, 50, 14843–14846. [Google Scholar] [CrossRef]
- Mamiya, J.-I.; Yoshitake, A.; Kondo, M.; Yu, Y.; Ikeda, T. Is chemical crosslinking necessary for the photoinduced bending of polymer films? J. Mater. Chem. 2008, 18, 63–65. [Google Scholar] [CrossRef]
- Aoki, K.I.; Nakagawa, M.; Ichimura, K. Self-assembly of amphoteric azopyridine carboxylic acids: Organized structures and macroscopic organized morphology influenced by heat, pH change, and light. J. Am. Chem. Soc. 2000, 122, 10997–11004. [Google Scholar] [CrossRef]
- Alaasar, M.; Tschierske, C.; Prehm, M. Hydrogen-bonded supramolecular complexes formed between isophthalic acid and pyridine-based derivatives. Liq. Cryst. 2011, 38, 925–934. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M. Mesophase behaviour of azobenzene-based angular supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2016, 43, 222–234. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Refaie, A.A.; Alaasar, M.A. Novel hydrogen-bonded angular supramolecular liquid crystals. Liq. Cryst. 2012, 39, 47–61. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Alaasar, M. Supramolecular hydrogen-bonded liquid crystals formed from 4-(4’-pyridylazophenyl)-4”-alkoxy benzoates and 4-substituted benzoic acids. Mol. Cryst. Liq. Cryst. 2008, 487, 74–91. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Alaasar, M. Supramolecular liquid crystals induced by hydrogen-bonding interactions between non-mesomorphic compounds. I. 4-(4’-pyridylazophenyl)-4”-substituted benzoates and 4-substituted benzoic acids. Mol. Cryst. Liq. Cryst. 2009, 506, 22–33. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.G.A.; Almllal, W.A. Supramolecular liquid crystals induced by hydrogen-bonding interactions between non-mesomorphic compounds. II. effect of lateral substitution. Mol. Cryst. Liq. Cryst. 2010, 518, 109–128. [Google Scholar] [CrossRef]
- Carli, J.T.; Lindberg, C.D.; Heltne, M.D.; Bornowski, E.C.; John, E.A.; Wiegel, K.N. Supramolecular main-chain liquid crystalline polymers with competitive hydrogen bonding: Inclusion of structurally analogous hydrogen bond acceptors and the effects on liquid crystallinity. Mol. Cryst. Liq. Cryst. 2017, 656, 83–88. [Google Scholar] [CrossRef]
- Mallia, V.A.; George, M.; Das, S. Photochemical phase transition in hydrogen-bonded liquid crystals. Chem. Mater. 1999, 11, 207–208. [Google Scholar] [CrossRef]
- Chen, K.-Y. Crystal structure, hydrogen-bonding properties, and DFT studies of 2-((2-(2-hydroxyphenyl) benzo [D] thiazol-6-Yl) methylene) malononitrile. Mol. Cryst. Liq. Cryst. 2015, 623, 285–296. [Google Scholar] [CrossRef]
- Shoji, M.; Tanaka, F. Theoretical study of hydrogen-bonded supramolecular liquid crystals. Macromolecules 2002, 35, 7460–7472. [Google Scholar] [CrossRef]
- Sundaram, S.; Jayaprakasam, R.; Dhandapani, M.; Senthil, T.; Vijayakumar, V. Theoretical (DFT) and experimental studies on multiple hydrogen bonded liquid crystals comprising between aliphatic and aromatic acids. J. Mol. Liq. 2017, 243, 14–21. [Google Scholar] [CrossRef]
- Hagar, M.; Soliman, S.M.; Ibid, F.; El Sayed, H. Quinazolin-4-Yl-sulfanylacetyl-hydrazone derivatives; synthesis, molecular structure and electronic properties. J. Mol. Struct. 2013, 1049, 177–188. [Google Scholar] [CrossRef]
- Soliman, S.M.; Hagar, M.; Ibid, F.; El Sayed, H. Experimental and theoretical spectroscopic studies, homo–lumo, nbo analyses and thione–thiol tautomerism of a new hybrid of 1,3,4-oxadiazole-thione with quinazolin-4-one. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Hagar, M.; Soliman, S.M.; Ibid, F.; El Sayed, H. Synthesis, molecular structure and spectroscopic studies of some new quinazolin-4 (3h)-one derivatives: An account on the N-versus S-alkylation. J. Mol. Struct. 2016, 1108, 667–679. [Google Scholar] [CrossRef]
- Aboelnaga, A.; Hagar, M.; Soliman, S.M. Ultrasonic synthesis, molecular structure and mechanistic study of 1, 3-dipolar cycloaddition reaction of 1-alkynylpyridinium-3-olate and acetylene derivatives. Molecules 2016, 21, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, H.A.; Hagar, M.; El-Sayed, T.H.; Alnoman, R.B. Schiff base/ester liquid crystals with different lateral substituents: Mesophase behaviour and DFT calculations. Liq. Cryst. 2019, 46, 1–11. [Google Scholar] [CrossRef]
- Paterson, D.A.; Gao, M.; Kim, Y.-K.; Jamali, A.; Finley, K.L.; Robles-Hernández, B.; Diez-Berart, S.; Salud, J.; De La Fuente, M.R.; Timimi, B.A. Understanding the twist-bend nematic phase: The characterisation of 1-(4-cyanobiphenyl-4’-yloxy)-6-(4-cyanobiphenyl-4’-yl) hexane (Cb6ocb) and comparison with Cb7cb. Soft Matter 2016, 12, 6827–6840. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Felipe, A.; Cook, A.G.; Abberley, J.P.; Walker, R.; Storey, J.M.; Imrie, C.T. An FT-IR spectroscopic study of the role of hydrogen bonding in the formation of liquid crystallinity for mixtures containing bipyridines and 4-pentoxybenzoic acid. RSC Adv. 2016, 6, 108164–108179. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.; Hagar, M.; Alaasar, M.; Naoum, M. Wide nematic phases induced by hydrogen-bonding. Liq. Cryst. 2019, 46, 550–559. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Aljuhani, A. Mesophase behavior of new linear supramolecular hydrogen-bonding complexes. RSC Adv. 2018, 8, 34937–34946. [Google Scholar] [CrossRef] [Green Version]
- Alnoman, R.B.; Ahmed, H.A.; Hagar, M.; Al-Ola, A.; Khulood, A.; Alrefay, B.S.; Haddad, B.A.; Albalawi, R.F.; Aljuhani, R.H.; Aloqebi, L.D. Induced phases of new H-bonded supramolecular liquid crystal complexes; mesomorphic and geometrical estimation. Molecules 2020, 25, 1549. [Google Scholar] [CrossRef] [Green Version]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; Alhaddad, O.; El-Shishtawy, R.M.; Raffah, B.M. New two rings schiff base liquid crystals; ball mill synthesis, mesomorphic, hammett and DFT studies. J. Mol. Liq. 2020, 299, 112161. [Google Scholar] [CrossRef]
- Ahmed, N.H.; Saad, G.R.; Ahmed, H.A.; Hagar, M. New wide-stability four-ring azo/ester/schiff base liquid crystals: Synthesis, mesomorphic, photophysical, and DFT approaches. RSC Adv. 2020, 10, 9643–9656. [Google Scholar] [CrossRef] [Green Version]
- Alnoman, R.; Ahmed, H.A.; Hagar, M.; Abu Al-Ola, K.; Alrefay, B.; Haddad, B.; AlBalawi, R.; Aljuhani, R.; Aloqebi, L.; AlSenani, S. Induced Phases of New H-bonded Supramolecular Liquid Crystal Complexes; Mesomorphic and Geometrical Estimation. Molecules 2020, 25, 1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mutabagani, L.A.; Alshabanah, L.A.; Ahmed, H.A.; Hagar, M.; Al-Ola, K.A.A. New symmetrical U-and wavy-shaped supramolecular H-bonded systems; geometrical and mesomorphic approaches. Molecules 2020, 25, 1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhaddad, O.A.; Ahmed, H.A.; Hagar, M.; Saad, G.R.; Al-Ola, A.; Khulood, A.; Naoum, M.M. Thermal and photophysical studies of binary mixtures of liquid crystal with different geometrical mesogens. Crystals 2020, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.; Hagar, M.; Alhaddad, O. Mesomorphic and geometrical orientation study of the relative position of fluorine atom in some thermotropic liquid crystal systems. Liq. Cryst. 2020, 47, 404–413. [Google Scholar] [CrossRef]
- Dave, J.S.; Menon, M. Azomesogens with a heterocyclic moiety. Bull. Mater. Sci. 2000, 23, 237–238. [Google Scholar] [CrossRef]
- Abberley, J.P.; Killah, R.; Walker, R.; Storey, J.M.; Imrie, C.T.; Salamończyk, M.; Zhu, C.; Gorecka, E.; Pociecha, D. Heliconical smectic phases formed by achiral molecules. Nat. Commun. 2018, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Paterson, D.A.; Crawford, C.A.; Pociecha, D.; Walker, R.; Storey, J.M.; Gorecka, E.; Imrie, C.T. The role of a terminal chain in promoting the twist-bend nematic phase: The synthesis and characterisation of the 1-(4-cyanobiphenyl-4’-yl)-6-(4-alkyloxyanilinebenzylidene-4’-oxy) hexanes. Liq. Cryst. 2018, 45, 2341–2351. [Google Scholar] [CrossRef]
- Stoimenovski, J.; Dean, P.M.; Izgorodina, E.I.; Macfarlane, D.R. Protic pharmaceutical ionic liquids and solids: Aspects of protonics. Faraday Discuss. 2012, 154, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Pibiri, I.; Pace, A.; Buscemi, S.; Causin, V.; Rastrelli, F.; Saielli, G. Oxadiazolyl-pyridines and perfluoroalkyl-carboxylic acids as building blocks for protic ionic liquids: Crossing the thin line between ionic and hydrogen bonded materials. Phys. Chem. Chem. Phys. 2012, 14, 14306–14314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dega-Szafran, Z.; Dulewicz, E. Infrared and 1 H nuclear magnetic resonance studies of hydrogen bonds in some pyridine trifluoroacetates and their deuteriated analogues in dichloromethane. J. Chem. Soc. Perkin Trans. 2 1983, 3, 345–351. [Google Scholar] [CrossRef]
- Cleland, W.; Kreevoy, M.M. Low-barrier hydrogen bonds and enzymic catalysis. Science 1994, 264, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Lizu, M.; Lutfor, M.; Surugau, N.; How, S.; Arshad, S.E. Synthesis and characterization of ethyl cellulose–based liquid crystals containing azobenzene chromophores. Mol. Cryst. Liq. Cryst. 2010, 528, 64–73. [Google Scholar] [CrossRef]
- Martínez-Felipe, A.; Imrie, C.T. The role of hydrogen bonding in the phase behaviour of supramolecular liquid crystal dimers. J. Mol. Struct. 2015, 1100, 429–437. [Google Scholar] [CrossRef]
- Ghanem, A.; Noel, C. FTIR investigation of two alkyl-p-terphenyl-4,4”-dicarboxylates in their crystalline, smectic and isotropic phases. Mol. Cryst. Liq. Cryst. 1987, 150, 447–472. [Google Scholar] [CrossRef]
- Paterson, D.A.; Martínez-Felipe, A.; Jansze, S.M.; Tm Marcelis, A.; Md Storey, J.; Imrie, C.T. New insights into the liquid crystal behaviour of hydrogen-bonded mixtures provided by temperature-dependent FTIR spectroscopy. Liq. Cryst. 2015, 42, 928–939. [Google Scholar] [CrossRef]
- Walker, R.; Pociecha, D.; Abberley, J.; Martinez-Felipe, A.; Paterson, D.; Forsyth, E.; Lawrence, G.; Henderson, P.; Storey, J.; Gorecka, E. Spontaneous chirality through mixing achiral components: A twist-bend nematic phase driven by hydrogen-bonding between unlike components. Chem. Commun. 2018, 54, 3383–3386. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M.; Saad, G. Mesophase behaviour of 1: 1 mixtures of 4-n-alkoxyphenylazo benzoic acids bearing terminal alkoxy groups of different chain lengths. Liq. Cryst. 2016, 43, 1259–1267. [Google Scholar] [CrossRef]
- Imrie, C.; Taylor, L. The preparation and properties of low molar mass liquid crystals possessing lateral alkyl chains. Liq. Cryst. 1989, 6, 1–10. [Google Scholar] [CrossRef]
- Imrie, C.T. Non-symmetric liquid crystal dimers: How to make molecules intercalate. Liq. Cryst. 2006, 33, 1449–1485. [Google Scholar] [CrossRef]
- Nafee, S.S.; Ahmed, H.A.; Hagar, M. New architectures of supramolecular H-bonded liquid crystal complexes based on dipyridine derivatives. Liq. Cryst. 2020, 1–14. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, G.; Zhong, X.; Yu, Y.; Gan, W. Effect of Pi–Pi stacking on the self-assembly of azomethine-type rod–coil liquid crystals. Liq. Cryst. 2011, 38, 995–1006. [Google Scholar] [CrossRef]
- Zang, L.; Che, Y.; Moore, J.S. One-dimensional self-assembly of planar Π-conjugated molecules: Adaptable building blocks for organic nanodevices. Acc. Chem. Res. 2008, 41, 1596–1608. [Google Scholar] [CrossRef]
- Scherf, U.; Adamczyk, S.; Gutacker, A.; Koenen, N. All-conjugated, rod-rod block copolymers-generation and self-assembly properties. Macromol. Rapid Commun. 2009, 30, 1059–1065. [Google Scholar] [CrossRef]
- He, G.; Li, Y.; Liu, J.; Yang, Y. Enhanced electroluminescence using polystyrene as a matrix. Appl. Phys. Lett. 2002, 80, 4247–4249. [Google Scholar] [CrossRef] [Green Version]
- Naoum, M.M.; Fahmi, A.A.; Mohammady, S.Z.; Abaza, A.H. Effect of lateral substitution on supramolecular liquid crystal associates induced by hydrogen-bonding interactions between 4-(4’-pyridylazo-3-methylphenyl)-4”-alkoxy benzoates and 4-substituted benzoic acids. Liq. Cryst. 2010, 37, 475–486. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, H.; Yan, M.; Guo, H.; Yang, F. The novel rufigallol-based liquid crystals with cholesterol units: Synthesis, mesomorphic and photophysical properties. Liq. Cryst. 2019, 46, 787–796. [Google Scholar] [CrossRef]
- Lin, L.; Qin, W.; Cheng, B.; Guo, H.; Yang, F. The influence of multiple alkyl chains on mesomorphic and photophysical properties of diphenylacrylonitrile liquid crystals. Liq. Cryst. 2019. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Z.; Li, X.; Yuan, Y.; Zhang, H. Influence of flexible spacer length on self-organization behaviors and photophysical properties of hemiphasmidic liquid crystalline polymers containing cyanostilbene. Eur. Polym. J. 2020, 123, 109459. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds I6-12/B are available from the authors. |













| System | TCr-N | ΔHCr-N | TN-I | ΔHN-I | ΔS/R |
|---|---|---|---|---|---|
| I6/B | 168.5 | 47.65 | 241.2 | 4.8 | 1.12 |
| I8/B | 153.1 | 57.38 | 237.4 | 5.43 | 1.28 |
| I10/B | 163.8 | 61.49 | 234.0 | 6.08 | 1.44 |
| I12/B | 169.2 | 64.08 | 230.1 | 8.57 | 2.05 |
| Parameter | I6/B | I8/B | I10/B | I12/B | II8/B |
|---|---|---|---|---|---|
| Ecorr | 1.025964 | 1.140069 | 1.254160 | 1.368322 | 0.959290 |
| ZPVE | −3168.161489 | −3325.295728 | −3482.429991 | −3639.564172 | −2644.501692 |
| Etot | −3168.093627 | −3325.222380 | −3482.351174 | −3639.479910 | −2644.441531 |
| H | −3168.092683 | −3325.221436 | −3482.350230 | −3639.478966 | −2644.440586 |
| G | −3168.288686 | −3325.431743 | −3482.573992 | −3639.716414 | −2644.616210 |
| Parameter | I6/B | I8/B | I10/B | I12/B | II8/B |
|---|---|---|---|---|---|
| ELUMO | −0.12678 | −0.12686 | −0.12703 | −0.12704 | −0.12450 |
| EHOMO | −0.22048 | −0.22039 | −0.22028 | −0.22028 | −0.22693 |
| ΔEHOMO-LUMO | 0.0937 | 0.09353 | 0.09325 | 0.09324 | 0.10243 |
| 1/ΔE | 10.67236 | 10.69176 | 10.72386 | 10.72501 | 9.762765 |
| μ Total | 1.7921 | 1.7962 | 1.7948 | 1.7348 | 2.6037 |
| Polarizability α | 901.07 | 949.74 | 997.65 | 1045.29 | 661.95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah Alshabanah, L.; Al-Mutabagani, L.A.; Ahmed, H.A.; Hagar, M. Induced Wide Nematic Phase by Seven-Ring Supramolecular H-Bonded Systems: Experimental and Computational Evaluation. Molecules 2020, 25, 1694. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071694
Abdullah Alshabanah L, Al-Mutabagani LA, Ahmed HA, Hagar M. Induced Wide Nematic Phase by Seven-Ring Supramolecular H-Bonded Systems: Experimental and Computational Evaluation. Molecules. 2020; 25(7):1694. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071694
Chicago/Turabian StyleAbdullah Alshabanah, Latifah, Laila A. Al-Mutabagani, Hoda A. Ahmed, and Mohamed Hagar. 2020. "Induced Wide Nematic Phase by Seven-Ring Supramolecular H-Bonded Systems: Experimental and Computational Evaluation" Molecules 25, no. 7: 1694. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071694