New Frontiers for the Use of IP6 and Inositol Combination in Treating Diabetes Mellitus: A Review
Abstract
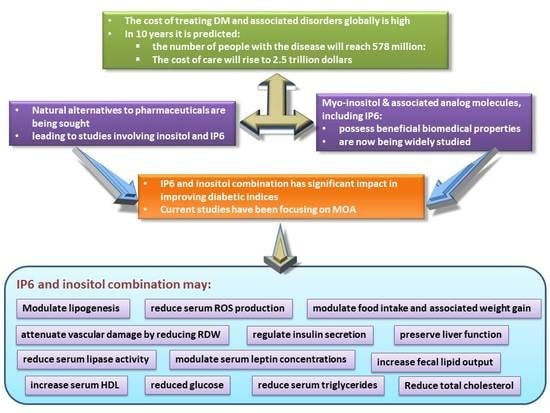
:1. Introduction
2. Inositol
3. Myo-Inositol Hexakisphosphate
4. Hypoglycemic Activity of IP6 and Inositol Supplement
5. Inositol and IP6 Supplementation and Serum Leptin
6. Inositol and IP6 Supplementation on Complete Blood Count
7. Inositol and IP6 Supplementation and Blood Lipids
8. Inositol and IP6 Administration and Organ Function
9. Intestinal Enzymes and Inositol and IP6 Supplementation
10. Inositol and IP6 Supplementation and Carbohydrate and Lipid Metabolism
11. Conclusions
Funding
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas—7th Edition. Available online: http://www.diabetesatlas.org/ (accessed on 19 January 2020).
- World Health Organization. Global Status Report on Noncommunicable Diseases; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watala, C.; Golański, J.; Boncler, M.A.; Pietrucha, T.; Gwoździński, K. Membrane Lipid Fluidity of Blood Platelets: A Common Denominator Underlying the Opposite Actions of Various Agents Affecting Platelet Activation in Whole Blood. Platelets 1998, 9, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Lu, W.; Jia, C.; Li, H.; Wang, Z.; Jia, W. Relationships between Glucose Excursion and the Activation of Oxidative Stress in Patients with Newly Diagnosed Type 2 Diabetes or Impaired Glucose Regulation. Endocrine 2010, 37, 201–208. [Google Scholar] [CrossRef]
- Augustyniak, K.; Zavodnik, I.; Palecz, D.; Szosland, K.; Bryszewska, M. The Effect of Oxidizing Agent and Diabetes Mellitus on the Human Red Blood Cell Membrane Potential. Clin. Biochem. 1996, 29, 283–286. [Google Scholar] [CrossRef]
- Zavodnik, I.B.; Szosaland, K.; Bryszewska, M. Human Red Blood Cell membrane potential and fluidity in glucose solutions. Scand. J. Clin. Lab. Investig. 1997, 57, 59–63. [Google Scholar] [CrossRef]
- Sözmen, E.Y.; Sözmen, B.; Delen, Y.; Onat, T. Catalase/Superoxide Dismutase (SOD) and Catalase/Paraoxonase (PON) Ratios May Implicate Poor Glycemic Control. Arch. Med. Res. 2001, 32, 283–287. [Google Scholar] [CrossRef]
- Narhe, S.; Kshirsagar, S.S.; Patil, V.S. Review on Medicinal Herbs Used for Diabetes. IJPMR 2018, 10, 224–228. [Google Scholar]
- Chhetri, D.R. Myo-Inositol and Its Derivatives: Their Emerging Role in the Treatment of Human Diseases. Front. Pharmacol. 2019, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, A.L.; Johnson, M.D.; Henry, S.A. 1L–myo-Inositol-1-phosphate synthase. Biochim. Biophys. Acta. 1997, 1348, 245–256. [Google Scholar] [CrossRef]
- Pereira, G.R.; Baker, L.; Egler, J.; Corcoran, L.; Chiavacci, R. Serum Myoinositol Concentrations in Premature Infants Fed Human Milk, Formula for Infants, and Parenteral Nutrition. Am. J. Clin. Nutr. 1990, 51, 589–593. [Google Scholar] [CrossRef]
- Ju, S.; Shaltiel, G.; Shamir, A.; Agam, G.; Greenberg, M.L. Human 1-D-myo-inositol-3-phosphate synthase is functional in yeast. J. Biol. Chem. 2004, 279, 21759–21765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, S.; McWilliams, A.; leRiche, J.; MacAulay, C. A phase I study of myo-inositol for lung cancer chemoprevention. Cancer Eoidemiol. Biomarkers Prev. 2006, 15, 1526–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlomagno, G.; Unfer, V. Inositol safety: Clinical evidences. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 931–936. [Google Scholar] [PubMed]
- Clements, R.S.; Darnell, B. Myo-inositol content of common foods: Development of a high myo-inositol diet. Am. J. Clin. Nutr. 1980, 33, 1954–1967. [Google Scholar] [CrossRef] [Green Version]
- Mancini, M.; Andreassi, A.; Salvioni, M.; Pelliccione, F.; Mantellassi, G.; Banderali, G. Inositol(s) from Bench to Bedside in Endocrinology and Gynecology. Int. J. Endocrinol. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Majumder, A.L.; Biswas, B.B. Biology of Inositols and Phosphoinositides. In Subcellular Biochemistry; Springer: New York, NY, USA, 2006. [Google Scholar]
- Shamsuddin, A.M.; Ullah, A.; Chakravarthy, A.K. Inositol and Inositol Hexaphosphate Suppress Cell Proliferation and Tumor Formation in CD-1 Mice. Carcinogenesis 1989, 10, 1461–1463. [Google Scholar] [CrossRef]
- Vucenik, I.; Yang, G.Y.; Shamsuddin, A.M. Inositol hexaphosphate and inositol inhibit DMBA-induced rat mammary cancer. Carcinogenesis 1995, 5, 1055–1058. [Google Scholar] [CrossRef]
- Barker, C.J.; Leibiger, I.B.; Leibiger, B.; Berggren, P.O. Phosphorylated Inositol Compounds in Beta -Cell Stimulus-Response Coupling. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E1113–E1122. [Google Scholar] [CrossRef]
- Berggren, P.O.; Barker, C.J. A Key Role for Phosphorylated Inositol Compounds in Pancreatic Beta-Cell Stimulus-Secretion Coupling. Adv. Enzyme. Regul. 2008, 48, 276–294. [Google Scholar] [CrossRef]
- Barker, C.J.; Illies, C.; Fiume, R.; Gaboardi, G.C.; Yu, J.; Berggren, P.O. Diphosphoinositol Pentakisphosphate as a Novel Mediator of Insulin Exocytosis. Adv. Enzym. Regul. 2009, 49, 168–173. [Google Scholar] [CrossRef]
- Tengholm, A.; Gylfe, E. Oscillatory Control of Insulin Secretion. Mol. Cell. Endocrinol. 2009, 297, 58–72. [Google Scholar] [CrossRef] [Green Version]
- Jacel, J.Y.; DeRouchey, J.M.; Tokach, M.D.; Geodband, R.D.; Nelssen, J.L.; Renter, D.G.; Dritz, S.S. Feed Additives for Swine: Fact Sheets – Prebiotics ad Probiotics and Phytogentics. J. Swine Health. Prod. 2010, 18, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Somasundar, P.; Riggs, D.R.; Jackson, B.J.; Cunningham, C.; McFadden, D.W. Inositol Hexaphosphate (IP6): A Novel Treatment for Pancreatic Cancer. J. Surg. Res. 2005, 126, 199–203. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frolich, W.; Prieto, R.M.; Grases, F. Phytate in Foods and Significance for Humans: Food Sources, Intake, Processing, Bioavailability, Protective Role and Analysis. Mol. Nutr. Food. Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2015, 1, 5–29. [Google Scholar] [CrossRef]
- Larson, S.R.; Rutger, J.N.; Young, K.A.; Raboy, V. Isolation and Genetic Mapping of a Non-lethal Rice (Oryza sativa L.) Low Phytic Acid 1 Mutation. Crop Sci. 2000, 40, 1397–1405. [Google Scholar] [CrossRef] [Green Version]
- Konietzny, U.; Jany, K.D.; Greiner, R. Phytate—An Undesirable Constituent of Plant-based Foods? J. Ernahr. 2006, 8, 18–28. [Google Scholar]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Irvine, R.F.; Schell, M.J. Back in the Water: The Return of the Inositol Phosphates. Nat. Rev. Mol. Cell. Biol. 2001, 2, 327–338. [Google Scholar] [CrossRef]
- Sauer, K.; Cooke, M.P. Regulation of Immune Cell Development Through Soluble Inositol-1,3,4,5-tetrakisphosphate. Nat. Rev. Immunol. 2010, 10, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Shears, S.B. The Versatility of Inositol Phosphates as Cellular Signals. Biochim. Biophys. Acta 1998, 1436, 49–67. [Google Scholar] [CrossRef]
- Vucenik, I.; Passaniti, A.; Vitolo, M.I.; Tantivejkul, K.; Eggleton, P.; Shamsuddin, A.M. Anti-angiogenic Activity of Inositol Hexaphosphate (IP6). Carcinogenesis 2004, 25, 2115–2123. [Google Scholar] [CrossRef] [Green Version]
- Foster, S.R.; Omoruyi, F.O.; Bustamante, J.; Lindo, R.L.A.; Dilworth, L.L. The Effect of Combined Inositol Hexakisphosphate and Inositol Supplement in Streptozotocin-Induced Type 2 Diabetic Rats. Int. J. Exp. Pathol. 2016, 97, 397–407. [Google Scholar] [CrossRef]
- Matsunami, T.; Sato, Y.; Sato, T.; Ariga, S.; Shimomura, T.; Yukawa, M. Oxidative Stress and Gene Expression of Antioxidant Enzymes in the Streptozotocin-Induced Diabetic Rats under Hyperbaric Oxygen Exposure. Int. J. Clin. Exp. Pathol. 2010, 3, 177–188. [Google Scholar]
- Chakraborty, A.; Kim, S.; Snyder, S.H. Inositol Pyrophosphates as Mammalian Cell Signals. Sci. Signal. 2011, 4, re1. [Google Scholar] [CrossRef] [Green Version]
- Raboy, V. Approaches and Challenges to Engineering Seed Phytate and Total Phosphorus. Plant Sci. 2009, 177, 281–296. [Google Scholar] [CrossRef]
- Sparvoli, F.; Cominelli, E. Seed Biofortification and Phytic Acid Reduction: A Conflict of Interest for the Plant? Plants 2015, 4, 728–755. [Google Scholar] [CrossRef] [Green Version]
- Kishor, D.S.; Lee, C.; Lee, D.; Venkatesh, J.; Seo, J.; Chin, J.H.; Jin, Z.; Hong, S.K.; Ham, J.K.; Koh, H.J. Novel Allelic Variant of Lpa1 Gene Associated with a Significant Reduction in Seed Phytic Acid Content in Rice (Oryza sativa L.). PLoS ONE 2019, 14, e0209636. [Google Scholar] [CrossRef] [Green Version]
- Vanderlinden, K.; Vucenik, I. Too Good to Be True? Inositol + Cal Mag IP6; Bearing Marketing Communications Limited: Mississauga, ON, Canada, 2004. [Google Scholar]
- Omoruyi, F.O. Beneficial and Adverse Effects of Phytic Acid Extract from Sweet Potato (Ipomoea Batatas). In Biometals: Molecular Structures, Binding Properties and Applications; Nova Science Publishers: New York, NY, USA, 2010. [Google Scholar]
- French, P.J.; Bunce, C.M.; Stephens, L.R.; Lord, J.M.; McConnell, F.M.; Brown, G.; Creba, J.A.; Michell, R.H. Changes in the Levels of Inositol Lipids and Phosphates during the Differentiation of HL60 Promyelocytic Cells towards Neutrophils or Monocytes. Proc. Biol. Sci. 1991, 245, 193–201. [Google Scholar] [CrossRef]
- Jackson, T.R.; Hallam, T.J.; Downes, C.P.; Hanley, M.R. Receptor Coupled Events in Bradykinin Action: Rapid Production of Inositol Phosphates and Regulation of Cytosolic Free Ca2+ in a Neural Cell Line. EMBO J. 1987, 6, 49–54. [Google Scholar] [CrossRef]
- Li, G.; Pralong, W.F.; Pittet, D.; Mayr, G.W.; Schlegel, W.; Wollheim, C.B. Inositol Tetrakisphosphate Isomers and Elevation of Cytosolic Ca2+ in Vasopressin-Stimulated Insulin-Secreting RINm5F Cells. J. Biol. Chem. 1992, 267, 4349–4356. [Google Scholar]
- Minihane, A.M.; Rimbach, G. Iron Absorption and the Iron Binding and Anti-Oxidant Properties of Phytic Acid. Int. J. Food Sci. Tech. 2002, 37, 741–748. [Google Scholar] [CrossRef]
- Zhou, J.R.; Erdman, J.W. Phytic Acid in Health and Disease. Crit. Rev Food Sci. Nutr. 1995, 35, 495–508. [Google Scholar] [CrossRef]
- Sakamoto, K.; Vucenik, I.; Shamsuddin, A.M. [3H] phytic acid (inositol hexaphosphate is absorbed and distributed to various tissues in rats. J. Nutr. 1993, 123, 713–720. [Google Scholar] [CrossRef]
- Grases, F.; Simonet, B.M.; Priet, R.M.; March, J.G. Variation of Insp4, Insp5 and Insp6 levels in tissues and biological fluids depending on dietary phytate. J. Nutr. Biochem. 2001, 12, 595–601. [Google Scholar] [CrossRef]
- Grases, F.; Simonet, B.M.; Priet, R.M.; March, J.G. Phytate levels in diverse rat tissues: Influence of dietary phytate. Br. J. Nutr. 2001, 86, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Sanchis, P.; Rivera, R.; Berga, F.; Fortuny, R.; Adrover, M.; Costa-Bauza, A.; Grases, F.; Masmiquel, L. Phytate decreases formation of advanced glycation end-products in patients with type II diabetes: Randomized crossover trial. Sci. Rep. 2018, 8, 9619. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, E.O.; Gerez, J.R.; Hohmann, M.S.N.; Verri Jn, W.A.; Bracarense, A.F.R.L. Phytic acid decreases oxidative stress and intestinal lesions induced by fumonisin B1 and deoxynivalenol in intestinal explants of pigs. Toxins 2019, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Grases, F.; Costa-Bauza, A.; Berga, F.; Gomila, R.M.; Martorell, G.; Martinez-Cignoni, M.R. Intake of myo-inositol hexaphosphate and urinary excretion of inositol phosphates in Wistar rats: Gavage vs. oral administration with sugar. PLoS ONE 2019, 14, e0223959. [Google Scholar] [CrossRef]
- Grases, F.; Costa-Bauza, A. Key aspects of myo-inositol hexaphosphate and pathological calcifications. Molecules 2019, 24, 4434. [Google Scholar] [CrossRef] [Green Version]
- Ferry, S.; Matsuda, M.; Yoshida, H.; Hirata, M. Inositol hexakisphosphate blocks tumor cell growth by activating apoptotic machinery as well as by inhibiting the Akt/NFκB-mediated cell survival pathway. Carcinogenesis 2002, 23, 2031–2041. [Google Scholar] [CrossRef] [Green Version]
- Vucenik, I. Conundrum of IP6. Open Biol. 2015, 5, 150048. [Google Scholar] [CrossRef] [Green Version]
- Vucenik, I.; Shamsuddin, A. Cancer Inhibition by Inositol Hexaphosphate (IP6) and Inositol: From Laboratory to Clinic. J. Nutr. 2003, 133 (Suppl. 1), S3778–S3784. [Google Scholar] [CrossRef]
- Vucenik, I. Protection against Cancer by Dietary IP6 and Inositol. Nutr. Cancer 2006, 55, 109–125. [Google Scholar] [CrossRef]
- Shamsuddin, A.M.; Elsayed, A.M.; Ullah, A. Suppression of Large Intestinal Cancer in F344 Rats by Inositol Hexaphosphate. Carcinogenesis 1988, 9, 577–580. [Google Scholar] [CrossRef]
- Vucenik, I.; Shamsuddin, A.M. [3H] Inositol Hexaphosphate (Phytic Acid) Is Rapidly Absorbed and Metabolized by Murine and Human Malignant Cells in Vitro. J. Nutr. 1994, 124, 861–868. [Google Scholar] [CrossRef]
- Grases, F.; Simonet, B.M.; Vucenik, I.; Perelló, J.; Prieto, R.M.; Shamsuddin, A.M. Effects of Exogenous Inositol Hexakisphosphate (InsP6) on the Levels of InsP6 and of Inositol Trisphosphate (InsP3)) in Malignant Cells, Tissues and Biological Fluids. Life Sci. 2002, 71, 1535–1546. [Google Scholar] [CrossRef]
- Vucenik, I. Anticancer Properties of Inositol Hexaphosphate and Inositol: An Overview. J. Nutr. Sci. Vitaminol. 2019, 65, S18–S22. [Google Scholar] [CrossRef] [Green Version]
- Dilworth, L.L.; Omoruyi, F.O.; Simon, O.R.; Morrison, E.S.A.; Asemota, H.N. The Effect of Phytic Acid on the Levels of Blood Glucose and Some Enzymes of Carbohydrate and Lipid Metabolism. West Indian Med. J. 2005, 54, 102–106. [Google Scholar] [CrossRef]
- Berridge, M.J.; Irvine, R.F. Inositol Trisphosphate, a Novel Second Messenger in Cellular Signal Transduction. Nature 1984, 312, 315–321. [Google Scholar] [CrossRef]
- Irvine, R.F. Inositide evolution-toward turtle domination? J. Physiol. 2005, 566, 295–300. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, H.J.; Chun, H.K.; Cho, S.Y.; Cho, S.M.; Lillehoj, H.S. Dietary Phytic Acid Lowers the Blood Glucose Level in Diabetic KK Mice. Nutr. Res. 2006, 26, 474–479. [Google Scholar] [CrossRef]
- Omoruyi, F.O.; Budiaman, A.; Eng, Y.; Olumese, F.E.; Hoesel, J.L.; Ejilemele, A.; Okorodudu, A.O. The Potential Benefits and Adverse Effects of Phytic Acid Supplement in Streptozotocin-Induced Diabetic Rats. Adv. Pharmacol. Sci. 2013, 172494. [Google Scholar] [CrossRef] [Green Version]
- Thompson, L.U. Antinutrients and Blood Glucose. Food Technol. 1988, 42, 123–132. [Google Scholar]
- Liu, N.; Ru, Y.J.; Li, F.D.; Cowieson, A.J. Effect of Diet Containing Phytate and Phytase on the Activity and Messenger Ribonucleic Acid Expression of Carbohydrase and Transporter in Chickens. J. Anim. Sci. 2008, 86, 3432–3439. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, S.S.; Cheryan, M. Effect of Phytic Acid, Divalent Cations and Their Interactions on Alpha Amylase Activity. J. Food. Sci. 1984, 47, 2080–2081. [Google Scholar] [CrossRef]
- McGregor, G.P.; Desaga, J.F.; Ehlenz, K.; Fischer, A.; Heese, F.; Hegele, A.; Lammer, C.; Peiser, C.; Lang, R.E. Radiommunological measurement of leptin in plasma of obese and diabetic human subjects. Endocrinology 1996, 137, 1501–1504. [Google Scholar] [CrossRef]
- Schwartz, M.W. Brain Pathways Controlling Food Intake and Body Weight. Exp. Biol. Med. 2001, 226, 978–981. [Google Scholar] [CrossRef]
- Sahu, A. Leptin Signaling in the Hypothalamus: Emphasis on Energy Homeostasis and Leptin Resistance. Front. Endocrinol. 2004, 24, 225–253. [Google Scholar] [CrossRef]
- Ahima, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Flier, J.S. Role of Leptin in the Neuroendocrine Response to Fasting. Nature 1996, 382, 250–252. [Google Scholar] [CrossRef]
- Myers, M.G.; Olson, D.P. Central Nervous System Control of Metabolism. Nature 2012, 491, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Seeley, R.J.; Tschöp, M.H.; Woods, S.C.; Morton, G.J.; Myers, M.G. Cooperation between Brain and Islet in Glucose Homeostasis and Diabetes. Nature 2013, 503, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Halaas, J.L.; Gajiwala, K.S.; Maffei, M.; Cohen, S.L.; Chait, B.T.; Rabinowitz, D.; Lallone, R.L.; Burley, S.K.; Friedman, J.M. Weight-Reducing Effects of the Plasma Protein Encoded by the Obese Gene. Science 1995, 269, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.O.; Vahl, N.; Dall, R.; Christiansen, J.S. Resting Metabolic Rate in Healthy Adults: Relation to Growth Hormone Status and Leptin Levels. Metabolism 1998, 47, 1134–1139. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Keogh, J.M.; Kamath, S.; Jones, S.; Gibson, W.T.; Trussell, R.; Jebb, S.A.; Lip, G.Y.; O’Rahilly, S. Partial Leptin Deficiency and Human Adiposity. Nature 2001, 2001. 414, 34–35. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Steadward, R.D.; Wheeler, G.D.; Bell, G.; McCargar, L.; Harber, V. Intact sympathetic nervous system is required for leptin effects on resting metabolic rate in people with spinal cord injury. J. Clin. Endocrinol. Metab. 2003, 88, 402–407. [Google Scholar] [CrossRef] [Green Version]
- Mărginean, C.O.; Mărginean, C.; Voidăzan, S.; Meliţ, L.; Crauciuc, A.; Duicu, C.; Bănescu, C. Correlations between Leptin Gene Polymorphisms 223 A/G 1019 G/A, 492 G/C, 976 C/A, and Anthropometrical and Biochemical Parameters in Children with Obesity: A Prospective Case-Control Study in a Romanian Population-the Nutrichild Study. Medicine 2016, 95, 3115. [Google Scholar] [CrossRef]
- Mărginean, C.; Mărginean, C.; Iancu, M.; Moldovan, V.; Meliț, L.; Bănescu, C. The Impact of TNF-α 308G>A Gene Polymorphism in Child’s Overweight Risk Coupled with the Assessment of Biochemical Parameters—A Cross-Sectional Single Center Experience. Pediatr. Neonatol. 2019, 60. [Google Scholar] [CrossRef] [Green Version]
- Mărginean, C.; Bănescu, C.; Duicu, C.; Pitea, A.; Voidăzan, S.; Mărginean, C. The Role of IL-6 572 C/G, 190 C/T, and 174 G/C Gene Polymorphisms in Children’s Obesity. Eur. J. Pediatr. 2014, 173, 1285–1296. [Google Scholar]
- Mărginean, C.O.; Bănescu, C.; Duicu, C.; Voidăzan, S.; Mărginean, C. Angiotensin Converting Enzyme Gene Insertion/Deletion Polymorphism in Nutritional Disorders in Children. Eur. J. Nutr. 2015, 54, 1245–1254. [Google Scholar] [CrossRef]
- Engeli, S.; Feldpausch, M.; Gorzelniak, K.; Hartwig, F.; Heintze, U.; Janke, J.; Möhlig, M.; Pfeiffer, A.F.H.; Luft, F.C.; Sharma, A.M. Association between Adiponectin and Mediators of Inflammation in Obese Women. Diabetes 2003, 52, 942–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoccali, C.; Mallamaci, F.; Tripepi, G.; Benedetto, F.A.; Cutrupi, S.; Parlongo, S.; Malatino, L.S.; Bonanno, G.; Seminara, G.; Rapisarda, F.; et al. Adiponectin, Metabolic Risk Factors, and Cardiovascular Events among Patients with End-Stage Renal Disease. J. Am. Soc. Nephrolog. 2002, 13, 134–141. [Google Scholar]
- Wiedmer, P.; Nogueiras, R.; Broglio, F.; D’Alessio, D.; Tschop, M.H. Ghrelin, obesity and diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 705–712. [Google Scholar] [CrossRef]
- Araujo-Vilar, D.; Santini, F. Diagnosis and Treatment of Lipodystrophy: A Step-by-Step Approach. J. Endocrinol. Investig. 2019, 42, 61–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diker-Cohen, T.; Cochran, E.; Gorden, P.; Brown, R.J. Partial and generalized lipodystrophy: Comparison of baseline characteristics and response to metreleptin. J. Clin. Endocrinol. Metab. 2015, 100, 1802–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oral, E.A.; Simha, V.; Ruiz, E.; Andewelt, A.; Premkumar, A.; Snell, P.; Wagner, A.J.; DePaoli, A.M.; Reitman, M.L.; Taylor, S.I.; et al. Leptin-Replacement Therapy for Lipodystrophy. N. Engl. J. Med. 2002, 346, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, A.; Ashida, K.; Moritaka, K.; Hidaka, M.; Gobaru, M.; Tanaka, S.; Hasuzawa, N.; Akasu, S.; Goto, Y.; Motomura, S.; et al. Metreleptin Supplementation for Improving Lipid and Glycemic Profiles in Acquired Diabetes Lipodystrophy: A Case Report. J. Endocr. Soc. 2019, 3, 2179–2183. [Google Scholar] [CrossRef] [Green Version]
- Seufert, J. Leptin Effects on Pancreatic Beta-Cell Gene Expression and Function. Diabetes 2004, 53 (Suppl. 1), S152–S158. [Google Scholar] [CrossRef] [Green Version]
- Soderberg, S.; Zimmet, P.; Tuomilehto, J.; Chitson, P.; Gareeboom, H.; Alberti, K.G.; Shaw, J.E. Leptin Predicts the Development of Diabetes in Mauritian Men, but Not Women: A Population-Based Study. Int. J. Obes 2007, 31, 1126–1133. [Google Scholar] [CrossRef] [Green Version]
- Toyoshima, Y.; Gavrilova, O.; Yakar, S.; Jou, W.; Pack, S.; Asghar, Z.; Wheeler, M.B.; LeRoith, D. Leptin Improves Insulin Resistance and Hyperglycemia in a Mouse Model of Type 2 Diabetes. Endocrinology 2005, 146, 4024–4035. [Google Scholar] [CrossRef] [Green Version]
- Yaspelkis, B.B.; Davis, J.R.; Saberi, M.; Smith, T.L.; Jazayeri, R.; Singh, M.; Fernandez, V.; Trevino, B.; Chinookoswong, N.; Wang, J.; et al. Leptin Administration Improves Skeletal Muscle Insulin Responsiveness in Diet-Induced Insulin-Resistant Rats. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E130–E142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Park, B.H.; Wang, M.Y.; Wang, Z.V.; Unger, R.H. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc. Natl. Acad. Sci. USA 2008, 105, 14070–14075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujikawa, T.; Chuang, J.C.; Sakata, I.; Ramadori, G.; Coppari, R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc. Natl. Acad. Sci. USA 2010, 107, 17391–17396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.N.; Han, S.N.; Kim, H.K. Phytic Acid and Myo-Inositol Support Adipocyte Differentiation and Improve Insulin Sensitivity in 3T3-L1 Cells. Nutr. Res. 2014, 34, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Malarvizhi, R.; Sali, V.K.; Kumari, M.; Vasanthi, H.R. Adipogenesis in Obesity Is Modulated by IP6 in Peanuts through Activation of the Nuclear Receptors (PPARs). J. Obes. Overweight 2016, 2, 103. [Google Scholar]
- Evans, T.C.; Jehle, D. The Red Blood Cell Distribution Width. J. Emerg. Med 1991, 9 (Suppl. S1), 71–74. [Google Scholar] [CrossRef]
- Saygin, M.; Ozturk, O.; Ozguner, M.F.; Akkaya, A.; Varol, E. Hematological Parameters as Predictors of Cardiovascular Disease in Obstructive Sleep Apnea Syndrome Patients. Angiology 2016, 67, 461–470. [Google Scholar] [CrossRef]
- Uemura, Y.; Shibata, R.; Takemoto, K.; Uchikawa, T.; Koyasu, M.; Watanabe, H.; Mitsuda, T.; Miura, A.; Imai, R.; Watarai, M.; et al. Elevation of Red Blood Cell Distribution Width during Hospitalization Predicts Mortality in Patients with Acute Decompensated Heart Failure. J. Cardiol. 2016, 67, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.E.; Kim, S.J.; Hwang, H.S.; Chung, S.; Yang, C.W.; Shin, S.J. Progressive Rise in Red Blood Cell Distribution Width Predicts Mortality and Cardiovascular Events in End-Stage Renal Disease Patients. PLoS ONE 2015, 10, e0126272. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, M.; Sacks, F.; Arnold, M.; Moye, L.; Davis, B.; Pfeffer, M. For the Cholesterol and Recurrent Events (CARE) Trial Investigators. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation 2008, 117, 163–168. [Google Scholar]
- Malandrino, N.; Wu, W.C.; Taveira, T.H.; Whitlatch, H.B.; Smith, R.J. Association between Red Blood Cell Distribution Width and Macrovascular and Microvascular Complications in Diabetes. Diabetologia 2012, 55, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atalay, H.; Boyuk, B.; Ates, M.; Guzel, S.; Celebi, A.; Ekizoglu, I. Red Cell Distribution Width and Acute Complications of Diabetes. Acta Endocrinologica 2018, 14, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goodnough, L.T. Anemia of Chronic Disease. New. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Moneim, A.; Abdel-Reheim, E.S.; Semmler, M.; Addaleel, W. The impact of glycemic status and metformin administration on red blood cell indices and oxidative stress in type 2 diabetic patients. Malays. J. Med. Sci. 2019, 26, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S. Red Cell Distribution Width, Inflammatory Markers and Cardiorespiratory Fitness: Results from the National Health and Nutrition Examination Survey. Indian Heart. J. 2012, 64, 380–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cakir, L.; Aktas, G.; Enginyurt, O.; Cakir, S.A. Mean Platelet Volume Increases in Type 2 Diabetes Mellitus Independent of HbA1c Level. Acta Med. Mediterr. 2014, 30, 425–428. [Google Scholar]
- Bhowmik, A.; Ojha, D.; Goswami, D.; Das, R.; Chandra, N.S.; Chatterjee, T.K.; Chakravarty, A.; Chakravarty, S.; Chattopadhyay, D. Inositol hexa phosphoric acid (phytic acid), A nutraceuticals, Attenuates iron-induced oxidative stress and alleviates liver injury in iron overloaded mice. Biomed. Pharmacother. 2017, 87, 443–450. [Google Scholar] [CrossRef]
- Foster, S.R.; Dilworth, L.L.; Thompson, R.K.; Alexander-Lindo, R.L.; Omoruyi, F.O. Effects of Combined Inositol Hexakisphosphate and Inositol Supplement on Antioxidant Activity and Metabolic Enzymes in the Liver of Streptozotocin-Induced Type 2 Diabetic Rats. Chem. Biol. Interact. 2017, 275, 108–115. [Google Scholar] [CrossRef]
- Liu, N.; Ru, Y.; Wang, J.; Xu, T. Effect of Dietary Sodium Phytate and Microbial Phytase on the Lipase Activity and Lipid Metabolism of Broiler Chickens. Br. J. Nutr. 2010, 103, 862–868. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Park, H.J.; Cho, S.Y.; Jung, H.J.; Cho, S.M.; Cho, Y.S.; Lillehoj, H.S. Effects of Dietary Phytic Acid on Serum and Hepatic Lipid Levels in Diabetic KK Mice. Nutr. Res 2005, 25, 869–876. [Google Scholar] [CrossRef]
- Yuangklang, C.; Wensing, T.; Lemmens, A.G.; Jittakhot, S.; Beynen, A.C. Effect of Sodium Phytate Supplementation on Fat Digestion and Cholesterol Metabolism in Female Rats. J. Anim. Physiol. Anim. Nutr. 2005, 89, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, L.L.; Omoruyi, F.O.; Asemota, H.N. Effects of IP6 and Sweet Potato (Ipomoea batatas) Phytate on Serum, Liver and Faecal Lipids in Rats. Int. J. Food Sci. Nutr. Eng. 2015, 5, 53–58. [Google Scholar]
- Pincus, M.R.; Sehaffner, J. R Assessment of Liver Function in Clinical Diagnosis and Management by Laboratory Methods; Saunders: Philadelphia, PA, USA, 1996. [Google Scholar]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.; Salam, S.K.; Salleh, M.S.; Gurtu, S. Comparison of antioxidant effects of honey, glibenclamide, metformin, and their combinations in the kidneys of streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2011, 12, 829–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarkli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Graf, E.; Mahoney, J.R.; Bryant, R.G.; Eaton, J.W. Iron Catalyzed Hydroxyl Radical Formation Stringent Requirement for Free Iron Coordination Site. J. Biol. Chem. 1984, 259, 3620–3624. [Google Scholar]
- Foster, S.R.; Dilworth, L.L.; Sparks, J.; Alexander-Lindo, R.L.; Omoruyi, F.O. Combined Inositol Hexakisphosphate and Inositol Supplement Consumption Improves Serum Alpha-Amylase Activity and Hematological Parameters in Streptozotocin-Induced Type 2 Diabetic Rats. Adv. Pharmacol. Sci. 2019, 4143137. [Google Scholar] [CrossRef] [Green Version]
- Urbano, G.; López-Jurado, M.; Aranda, P.; Vidal-Valverde, C.; Tenorio, E.; Porres, J. The Role Of phytic Acid in Legumes: Antinutrient or Beneficial Function? J. Physiol. Biochem. 2000, 56, 283–294. [Google Scholar] [CrossRef]
- Muzquiz, M.; Varela, A.; Burbano, C.; Cuadrado, C.; Guillamon, E.; Pedrosa, M.M. Bioactive Compounds in Legumes: Pronutritive and Antinutritive Actions. Implications for Nutrition and Health. Phytochem. Rev. 2012, 11, 227–244. [Google Scholar] [CrossRef]
- Aughsteen, A.A.; Abu-Umair, M.S.; Mahmoud, S.A. Biochemical Analysis of Serum Pancreatic Amylase and Lipase Enzymes in Patients with Type 1 and Type 2 Diabetes Mellitus. Saudi Med. J. 2005, 26, 73–77. [Google Scholar]
- Barreto, S.G.; Carati, C.J.; Toouli, J.; Saccone, G.T.P. The Islet-Acinar Axis of the Pancreas: More than Just Insulin. Am. J. Physiol.-Gastr. Liver Physiol. 2010, 299, G10–G22. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.; Bhartiya, J.P.; Verma, S.K.; Nandkeoliar, M.K. The Evaluation of Serum Amylase in the Patients of Type 2 Diabetes Mellitus, with a Possible Correlation with the Pancreatic Functions. J. Clin. Diagn. Res. 2013, 7, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Nemoto, T.; Muneyuki, T.; Kakei, M.; Fuchigami, H.; Munakata, H. Low serum amylase in association with metabolic syndrome and diabetes: A community-based study. Cardiovasc. Diabetol. 2011, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuppusamy, A.; Muthusamy, U.; Andichetiar Thirumalaisamy, S.; Varadharajan, S.; Ramasamy, K.; Ramanathan, S. In vitro (α-glucosidase and α-amylase inhibition) and in vivo antidiabetic property of phytic acid (IP6) in streptozotocin-nicotinamide- induced type 2 diabetes mellitus (NIDDM) in rats. J. Complement. Integr. Med. 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Omoruyi, F.; Adamson, I. Digestive and Hepatic Enzymes in Streptozotocin-Induced Diabetic Rats Fed Supplements of Dikanut (Irvingia Gabonensis) and Cellulose. Ann. Nutr. Metab. 1993, 37, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Onomi, S.; Okazaki, Y.; Katayama, T. Effect of Dietary Level of Phytic Acid on Hepatic and Serum Lipid Status in Rats Fed a High-Sucrose Diet. Biosci. Biotechnol. Biochem. 2004, 68, 1379–1381. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omoruyi, F.O.; Stennett, D.; Foster, S.; Dilworth, L. New Frontiers for the Use of IP6 and Inositol Combination in Treating Diabetes Mellitus: A Review. Molecules 2020, 25, 1720. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071720
Omoruyi FO, Stennett D, Foster S, Dilworth L. New Frontiers for the Use of IP6 and Inositol Combination in Treating Diabetes Mellitus: A Review. Molecules. 2020; 25(7):1720. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071720
Chicago/Turabian StyleOmoruyi, Felix O., Dewayne Stennett, Shadae Foster, and Lowell Dilworth. 2020. "New Frontiers for the Use of IP6 and Inositol Combination in Treating Diabetes Mellitus: A Review" Molecules 25, no. 7: 1720. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25071720