Cyclic Synthetic Peroxides Inhibit Growth of Entomopathogenic Fungus Ascosphaera apis without Toxic Effect on Bumblebees
Abstract
:1. Introduction
2. Results
2.1. Identification and Isolation of Ascosphaera apis
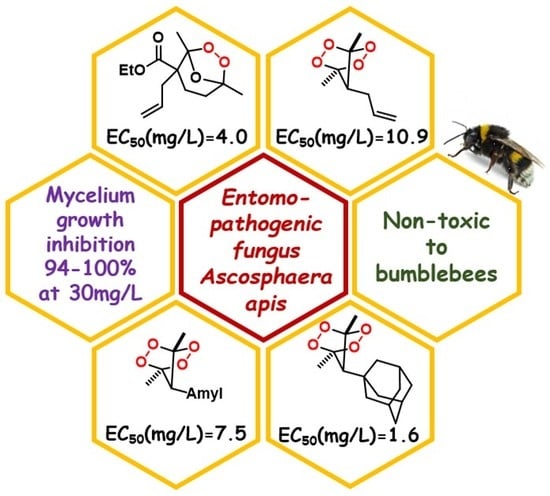
2.2. The Effect of the Peroxides on the Inhibition of Growth of Ascosphaera apis
2.3. Evaluation of Toxicity of Peroxides towards Bumblebees
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Molecular Identification of Microorganisms in the Intestinal Contents of Bumblebee Larvae
4.3. Study of the Toxic Effect of the Peroxides on Bumblebees (Contact Action)
4.4. Study of the Toxic Effect of the Peroxides on Bumblebees (Feeding of Bumblebees)
4.5. Study of the Ability of Bumblebees to Fly
4.6. Evaluation of Fungicidal Activity of the Peroxides
4.7. SEM Characterization of Ascosphaera apis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fisher, L.C.; Blackie, M.A. Tetraoxanes as antimalarials: Harnessing the endoperoxide. Mini-Rev. Med. Chem. 2014, 14, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, P.; Dussault, P.H.; Hu, C. Synthesis of spiro-bisperoxyketals. Org. Lett. 2008, 10, 2401–2404. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.D.; Wittlin, S.; Wu, Y. Potent antimalarial 1,2,4-trioxanes through perhydrolysis of epoxides. Chem. Eur. J. 2013, 19, 7605–7619. [Google Scholar] [CrossRef] [PubMed]
- Jefford, C.W. Synthetic Peroxides as Potent Antimalarials. News and Views. Curr. Top. Med. Chem. 2012, 12, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Opsenica, D.M.; Šolaja, B.A. Antimalarial peroxides. J. Serb. Chem. Soc. 2009, 74, 1155–1193. [Google Scholar] [CrossRef]
- Šolaja, B.A.; Terzić, N.; Pocsfalvi, G.; Gerena, L.; Tinant, B.; Opsenica, D.; Milhous, W.K.; Fisher, L.C.; Blackie, M.A. Mixed steroidal 1,2,4,5-tetraoxanes: Antimalarial and antimycobacterial activity. J. Med. Chem. 2014, 45, 3331–3336. [Google Scholar] [CrossRef]
- Cowan, N.; Yaremenko, I.A.; Krylov, I.B.; Terent’ev, A.O.; Keiser, J. Elucidation of the in vitro and in vivo activities of bridged 1,2,4-trioxolanes, bridged 1,2,4,5-tetraoxanes, tricyclic monoperoxides, silyl peroxides, and hydroxylamine derivatives against Schistosoma mansoni. Bioorgan. Med. Chem. 2015, 23, 5175–5181. [Google Scholar] [CrossRef] [Green Version]
- Ingram, K.; Yaremenko, I.A.; Krylov, I.B.; Hofer, L.; Terentev, A.O.; Keiser, J. Identification of antischistosomal leads by evaluating bridged 1,2,4,5-tetraoxanes, alphaperoxides, and tricyclic monoperoxides. J. Med. Chem. 2012, 55, 8700–8711. [Google Scholar] [CrossRef] [Green Version]
- Keiser, J.; Ingram, K.; Vargas, M.; Chollet, J.; Wang, X.; Dong, Y.; Vennerstrom, J.L. In vivo activity of aryl ozonides against Schistosoma species. Antimicrob. Agents Chemother. 2012, 56, 1090–1092. [Google Scholar] [CrossRef] [Green Version]
- Küster, T.; Kriegel, N.; Stadelmann, B.; Wang, X.; Dong, Y.; Vennerstrom, J.L.; Keiser, J.; Hemphill, A. Amino ozonides exhibit in vitro activity against Echinococcus multilocularis metacestodes. Int. J. Antimicrob. Agents 2014, 43, 40–46. [Google Scholar] [CrossRef]
- Vil’, V.A.; Yaremenko, I.A.; Ilovaisky, A.I.; Terent’ev, A.O. Peroxides with Anthelmintic, Antiprotozoal, Fungicidal and Antiviral Bioactivity: Properties, Synthesis and Reactions. Molecules 2017, 22, 1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrams, R.P.; Carroll, W.L.; Woerpel, K.A. Five-Membered Ring Peroxide Selectively Initiates Ferroptosis in Cancer Cells. ACS Chem. Biol. 2016, 11, 1305–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhari, M.B.; Moorthy, S.; Patil, S.; Bisht, G.S.; Mohamed, H.; Basu, S.; Gnanaprakasam, B. Iron-Catalyzed Batch/Continuous Flow C-H Functionalization Module for the Synthesis of Anticancer Peroxides. J. Org. Chem. 2018, 83, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Yaremenko, I.A.; Syroeshkin, M.A.; Levitsky, D.O.; Fleury, F.; Terent’ev, A.O. Cyclic peroxides as promising anticancer agents: In vitro cytotoxicity study of synthetic ozonides and tetraoxanes on human prostate cancer cell lines. Med. Chem. Res. 2017, 26, 170–179. [Google Scholar] [CrossRef]
- Coghi, P.; Yaremenko, I.A.; Prommana, P.; Radulov, P.S.; Syroeshkin, M.A.; Wu, Y.J.; Gao, J.Y.; Gordillo, F.M.; Mok, S.; Wong, V.K.W.; et al. Novel Peroxides as Promising Anticancer Agents with Unexpected Depressed Antimalarial Activity. Chemmedchem 2018, 13, 902–908. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Coghi, P.; Prommana, P.; Qiu, C.; Radulov, P.S.; Qu, Y.; Belyakova, Y.Y.; Zanforlin, E.; Kokorekin, V.A.; Wu, Y.Y.J.; et al. Synthetic Peroxides Promote Apoptosis of Cancer Cells by Inhibiting P-Glycoprotein ABCB5. Chemmedchem 2020. [Google Scholar] [CrossRef]
- Dwivedi, A.; Mazumder, A.; du Plessis, L.; du Preez, J.L.; Haynes, R.K.; du Plessis, J. In vitro anti-cancer effects of artemisone nano-vesicular formulations on melanoma cells. Nanomed.-Nanotechnol. 2015, 11, 2041–2050. [Google Scholar] [CrossRef]
- Chou, S.W.; Marousek, G.; Auerochs, S.; Stamminger, T.; Milbradt, J.; Marschall, M. The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants. Antivir. Res 2011, 92, 364–368. [Google Scholar] [CrossRef]
- Efferth, T.; Romero, M.R.; Wolf, D.G.; Stamminger, T.; Marin, J.J.G.; Marschall, M. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 2008, 47, 804–811. [Google Scholar] [CrossRef] [Green Version]
- Reiter, C.; Frohlich, T.; Gruber, L.; Hutterer, C.; Marschall, M.; Voigtlander, C.; Friedrich, O.; Kappes, B.; Efferth, T.; Tsogoeva, S.B. Highly potent artemisinin-derived dimers and trimers: Synthesis and evaluation of their antimalarial, antileukemia and antiviral activities. Bioorgan. Med. Chem. 2015, 23, 5452–5458. [Google Scholar] [CrossRef]
- Chaudhary, S.; Sharma, V.; Jaiswal, P.K.; Gaikwad, A.N.; Sinha, S.K.; Puri, S.K.; Sharon, A.; Maulik, P.R.; Chaturvedi, V. Stable Tricyclic Antitubercular Ozonides Derived from Artemisinin. Org. Lett. 2015, 17, 4948–4951. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Walz, A.J.; Zhu, H.; Wu, C.; Moraski, G.; Möllmann, U.; Tristani, E.M.; Crumbliss, A.L.; Ferdig, M.T.; Checkley, L.; et al. Design, Synthesis, and Study of a Mycobactin−Artemisinin Conjugate That Has Selective and Potent Activity against Tuberculosis and Malaria. J. Am. Chem. Soc. 2011, 133, 2076–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.W.; Lei, H.S.; Fan, L.; Jiang, L.; Liu, J.; Peng, X.M.; Xu, X.R.; Chen, L.; Zhou, C.H.; Zou, Y.Y.; et al. Design, synthesis, and biological evaluation of dihydroartemisinin- fluoroquinolone conjugates as a novel type of potential antitubercular agents. Bioorgan. Med. Chem. Lett. 2014, 24, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- NobelPrize.org. Tu Youyou—Facts. Nobel Media AB 2020. Available online: https://www.nobelprize.org/prizes/medicine/2015/tu/facts/ (accessed on 22 April 2020).
- Yaremenko, I.A.; Radulov, P.S.; Belyakova, Y.Y.; Demina, A.A.; Fomenkov, D.I.; Barsukov, D.V.; Subbotina, I.R.; Fleury, F.; Terent’ev, A. Catalyst development for the synthesis of ozonides and tetraoxanes under heterogeneous conditions. Disclosure of an unprecedented class of fungicides for agricultural application. Chem. Eur. J. 2020, 26, 4734–4751. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemuller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Thomann, M.; Imbert, E.; Devaux, C.; Cheptou, P.O. Flowering plants under global pollinator decline. Trends Plant Sci. 2013, 18, 353–359. [Google Scholar] [CrossRef]
- Rhodes, C.J. Pollinator decline-an ecological calamity in the making? Sci. Prog. 2018, 101, 121–160. [Google Scholar] [CrossRef]
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Roy. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. R. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Bryden, J.; Gill, R.J.; Mitton, R.A.A.; Raine, N.E.; Jansen, V.A.A. Chronic sublethal stress causes bee colony failure. Ecol. Lett. 2013, 16, 1463–1469. [Google Scholar] [CrossRef] [Green Version]
- Tosi, S.; Nieh, J.C. A common neonicotinoid pesticide, thiamethoxam, alters honey bee activity, motor functions, and movement to light. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Burgio, G.; Nieh, J.C. A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu-Smart, J.; Spivak, M. Effects of neonicotinoid imidacloprid exposure on bumble bee (Hymenoptera: Apidae) queen survival and nest initiation. Environ. Entomol. 2018, 47, 55–62. [Google Scholar] [CrossRef]
- Tosi, S.; Nieh, J.C.; Sgolastra, F.; Cabbri, R.; Medrzycki, P. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc. Roy. Soc. B 2017, 284, 20171711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, A.; Coleman, C.; Hoffmann, C.; Fritz, B.; Rangel, J. The Synergistic Effects of Almond Protection Fungicides on Honey Bee (Hymenoptera: Apidae) Forager Survival. J. Econ. Entomol. 2017, 110, 802–808. [Google Scholar] [CrossRef]
- Raimets, R.; Karise, R.; Mand, M.; Kaart, T.; Ponting, S.; Song, J.M.; Cresswell, J.E. Synergistic interactions between a variety of insecticides and an ergosterol biosynthesis inhibitor fungicide in dietary exposures of bumble bees (Bombus terrestris L.). Pest Manag. Sci. 2018, 74, 541–546. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Yao, J.X.; Adamczyk, J.; Luttrell, R. Feeding toxicity and impact of imidacloprid formulation and mixtures with six representative pesticides at residue concentrations on honey bee physiology (Apis mellifera). PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Sgolastra, F.; Medrzycki, P.; Bortolotti, L.; Renzi, M.T.; Tosi, S.; Bogo, G.; Teper, D.; Porrini, C.; Molowny-Horas, R.; Bosch, J. Synergistic mortality between a neonicotinoid insecticide and an ergosterol-biosynthesis-inhibiting fungicide in three bee species. Pest Manag. Sci. 2017, 73, 1236–1243. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Corby-Harris, V.; DeJong, E.W.; Chambers, M.; Hidalgo, G. Honey bee gut microbial communities are robust to the fungicide PristineA (R) consumed in pollen. Apidologie 2017, 48, 340–352. [Google Scholar] [CrossRef] [Green Version]
- Steffan, S.A.; Dharampal, P.S.; Diaz-Garcia, L.; Currie, C.R.; Zalapa, J.; Hittinger, C.T. Empirical, Metagenomic, and Computational Techniques Illuminate the Mechanisms by which Fungicides Compromise Bee Health. J. Vis. Exp. 2017, 128, e54631. [Google Scholar] [CrossRef] [PubMed]
- Kakumanu, M.L.; Reeves, A.M.; Anderson, T.D.; Rodrigues, R.R.; Williams, M.A. Honey Bee Gut Microbiome Is Altered by In-Hive Pesticide Exposures. Front. Microbiol. 2016, 7, 1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syromyatnikov, M.Y.; Kokina, A.V.; Lopatin, A.V.; Starkov, A.A.; Popov, V.N. Evaluation of the toxicity of fungicides to flight muscle mitochondria of bumblebee (Bombus terrestris L.). Pestic. Biochem. Phys. 2017, 135, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.H.; Krainer, S.; Engert, A.; Schuehly, W.; Riessberger-Galle, U.; Crailsheim, K. Sublethal pesticide doses negatively affect survival and the cellular responses in American foulbrood-infected honeybee larvae. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Grassl, J.; Holt, S.; Cremen, N.; Peso, M.; Hahne, D.; Baer, B. Synergistic effects of pathogen and pesticide exposure on honey bee (Apis mellifera) survival and immunity. J. Invertebr. Pathol. 2018, 159, 78–86. [Google Scholar] [CrossRef]
- O’Neill, P.M.; Amewu, R.K.; Charman, S.A.; Sabbani, S.; Gnadig, N.F.; Straimer, J.; Fidock, D.A.; Shore, E.R.; Roberts, N.L.; Wong, M.H.L.; et al. A tetraoxane-based antimalarial drug candidate that overcomes PfK13-C580Y dependent artemisinin resistance. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Aufauvre, J.; Biron, D.G.; Vidau, C.; Fontbonne, R.; Roudel, M.; Diogon, M.; Vigues, B.; Belzunces, L.P.; Delbac, F.; Blot, N. Parasite-insecticide interactions: A case study of Nosema ceranae and fipronil synergy on honeybee. Sci. Rep. 2012, 2, 326. [Google Scholar] [CrossRef]
- Spiltoir, C.F. Life cycle of Ascosphaera Apis (Pericystis Apis). Am. J. Bot. 1955, 42, 501–508. [Google Scholar] [CrossRef]
- Zaghloul, O.; Mourad, A.K.; EI Kady, M.B.; Nemat, F.; Morsy, M.E. Assessment of losses in honey yield due to the chalkbrood disease, with reference to the determination of its economic injury levels in Egypt. Commun. Agric. Appl. Biol. Sci. 2005, 70, 703–714. [Google Scholar]
- Bailey, L.; Ball, B.V. The Treatment of Bee Diseases. In Honey Bee Pathology, 2nd ed.; Bailey, L., Ball, B.V., Eds.; Academic Press: London, UK, 1991; pp. 132–153. [Google Scholar]
- Přidal, A.; Sedláček, I.; Marvanová, L. Microbiology of Bombus terrestris L. larvae (Hymenoptera:Apoidea) from laboratory rearing. Acta Univ. Agric. Silvic. Mendel Brun. 1997, 45, 59–66. [Google Scholar]
- Maxfield-Taylor, S.A.; Mujic, A.B.; Rao, S. First Detection of the Larval Chalkbrood Disease Pathogen Ascosphaera apis (Ascomycota: Eurotiomycetes: Ascosphaerales) in Adult Bumble Bees. PLoS ONE 2015, 10, e0124868. [Google Scholar] [CrossRef]
- Pereira, K.D.; Meeus, I.; Smagghe, G. Honey bee-collected pollen is a potential source of Ascosphaera apis infection in managed bumble bees. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terent’ev, A.O.; Yaremenko, I.A.; Chernyshev, V.V.; Dembitsky, V.M.; Nikishin, G.I. Selective Synthesis of Cyclic Peroxides from Triketones and H2O2. J. Org. Chem. 2012, 77, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Metodicheskie Rekomendatsii po Opredeleniyu Fungitsidnoi Aktivnosti Novykh Soedinenii [Guidelines for Determination of Fungicidal Activity of New Compounds], Russian ed.; NIITEKhIM: Cherkassy, Ukraine, 1984; p. 32. (In Russian)
- Kachala, V.V.; Khemchyan, L.L.; Kashin, A.S.; Orlov, N.V.; Grachev, A.A.; Zalesskiy, S.S.; Ananikov, V.P. Target-oriented analysis of gaseous, liquid and solid chemical systems by mass spectrometry, nuclear magnetic resonance spectroscopy and electron microscopy. Russ. Chem. Rev. 2013, 82, 648–685. [Google Scholar] [CrossRef]
- Kashin, A.S.; Ananikov, V.P. A SEM study of nanosized metal films and metal nanoparticles obtained by magnetron sputtering. Russ. Chem. Bull. Int. Ed. 2011, 60, 2602–2607. [Google Scholar] [CrossRef]
Sample Availability: Samples of all tested compounds are available from the authors. |




| Compound | Mycelium Growth Inhibition (I), % |
|---|---|
| 1 | 94 |
| 2 | 100 |
| 3 | 100 |
| 4 | 66 |
| 5 | 49 |
| 6 | 94 |
| 7 | 53 |
| 8 | 25 |
| Triadimefon 9 | 91 |
| Kresoxim-methyl 10 | 88 |
| Compound | 1 | 2 | 3 | 6 | Triadimefon, 9 | Kresoxim-Methyl, 10 |
|---|---|---|---|---|---|---|
| EC50 (mg/L) ± SD | 4.0 ± 0.2 | 10.9 ± 0.7 | 7.5 ± 1.0 | 1.6 ± 0.1 | 7.1 ± 0.8 | <1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaremenko, I.A.; Syromyatnikov, M.Y.; Radulov, P.S.; Belyakova, Y.Y.; Fomenkov, D.I.; Popov, V.N.; Terent’ev, A.O. Cyclic Synthetic Peroxides Inhibit Growth of Entomopathogenic Fungus Ascosphaera apis without Toxic Effect on Bumblebees. Molecules 2020, 25, 1954. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25081954
Yaremenko IA, Syromyatnikov MY, Radulov PS, Belyakova YY, Fomenkov DI, Popov VN, Terent’ev AO. Cyclic Synthetic Peroxides Inhibit Growth of Entomopathogenic Fungus Ascosphaera apis without Toxic Effect on Bumblebees. Molecules. 2020; 25(8):1954. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25081954
Chicago/Turabian StyleYaremenko, Ivan A., Mikhail Y. Syromyatnikov, Peter S. Radulov, Yulia Yu. Belyakova, Dmitriy I. Fomenkov, Vasily N. Popov, and Alexander O. Terent’ev. 2020. "Cyclic Synthetic Peroxides Inhibit Growth of Entomopathogenic Fungus Ascosphaera apis without Toxic Effect on Bumblebees" Molecules 25, no. 8: 1954. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25081954